 Refresh/Reload screen to see updates
Refresh/Reload screen to see updates|
|
 |
CHM130 (SUN#1130)
|
|
Class# 13418 |
INSTRUCTOR: Ken Costello chm130pc@chemistryland.com, (480)202-2993 |
| Photos of him impersonating famous chemists. | |
To see assignment scores, go to the saplinglearning.com gradebook. |
|
| If taking the on-campus CHM130 lab, you might find the below link to the help pages useful. If taking the online CHM130 lab, then no need to use these help pages. http://www.chemistryland.com/CHM130S/LabHelp/MenuForLabHelp.html | |
|
Syllabus and Sapling Learning Account |
 |
||
Target Dates My class doesn't have deadlines, but it has target dates for the quizzes and exams. Those are listed in Sapling Learning within the title of the quiz/exam. Target dates are set to keep you on target in completely the course at an even pace. If you do a quiz after its target date, the quiz is not late nor do you get penalized. It just means you are getting behind. |
 |
||
Student Information Sheet It's always helpful for instructors to have extra information about why you are taking the course and other relevant information. So we have a Student Information Sheet that we would like you to fill out. Here is the form as a Word document. Also, here is a Web page that has the sheet which you can copy and place in an email. Then add the information. |
|||
|
Why Chemistry? Throughout human history, chemistry has helped us survive. Turning stones into tools, making fire, creating ceramics, and preserving food were some of the earliest benefits of chemistry. Early
Chemistry Menu Page |
 |
||
Pitfalls to Learning Learn the pitfalls you encounter when
learning a difficult subject like chemistry No Quiz |
 |
||
|
Chemistry in a New Light Chemistry
in a New Light Tutorial |
 |
||
|
Scientific Skepticism Learning science means how to look beyond the surface, beyond the moment, and beyond the expected response. This approach helps us uncover the real truths behind what is presented. It also protects us from being fooled. Scientific Skepticism Menu Page (Tutorials & Quiz) |
 |
||
|
Mathematical
Universe Tamed |
 |
||
|
THE ENGLISH SYSTEM |
 |
||
|
Significant
Figures Tutorial & Reading Assignment |
|||
|
DIMENSIONAL
ANALYSIS tutorial Quiz based on end of Chapter 2 problems at Sapling Learning |
 |
||
|
|
||
 |
Building
Block Intro |
 |
Protons,
Electrons, and Neutrons Built from Energy |
|
|
|
|
||
|
|
||
Building
Blocks for Inorganic Compounds |
|
|
Building
Blocks for Organic Compounds |
|
|
CLASSIFYING:
CALMING THE CHAOS Quiz for Classifying: Calming the Chaos at Sapling Learning Extra Credit Quiz for Classifying Matter at Sapling Learning Extra Credit Quiz for Element names/formulas at Sapling Learning |
 |
|
Chapter 3: Elements and Compounds Quiz based on end of Chapter 3 problems at Sapling Learning |
 |
|
|
Physical and Chemical Properties Quiz for Physical and Chemical Properties & Changes at Sapling Learning |
 |
|
 |
||
|
Early
Development of Atomic Theory Chapter
5: Reading Assignment for Textbook |
 |
|
|
Quiz uses some Problems Pulled from Textbook (Chapter 5) |
 |
|
|
Nomenclature
Introduction & Menu
|
Midterm Exam |
|
|
The Art of Counting without Counting tutorial When items are too many to count or too small to count,
|
 |
|
|
Textbook: Read sections 7.1(Mole) and 7.2(Molar Mass) in chapter 7 The
Art of Counting Quiz at Sapling Learning |
 |
|
|
Writing
& Balancing Chemical Equations tutorial Reading for Writing & Balancing Chemical Equations:
|
 |
|
|
Balancing
Equations: Textbook Assignment as Quiz at Sapling Learning These quiz questions were pulled from question 11, p.170, 11th edition; question 3, p.172 from 12th edition question 17, p.171, 11th edition, and question 7, p.172, 12th edition |
||
|
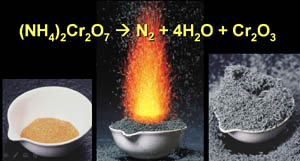
Types
of Chemical Reactions & their Chemical Equations tutorial (4/2/16) Reading for various editions: 11th &12th pp159-164, 13th pp151-157, 14th pp150-156 Types of Reactions Quiz at Sapling Learning |
 |
|
|
Stoichiometry tutorial Reading for various editions: 11th pp174-189, 12th pp176-191, 13th pp168-184, 14th pp150-156 |
 |
|
|
The Nature of Light and a Modern View of the Atom) This is a tutorial that shows how the electron structure of the atom was discovered. The quiz questions are scattered throughout the tutorial. Quiz on Nature of Light and the Modern Atom at Sapling Learning |
 |
|
|
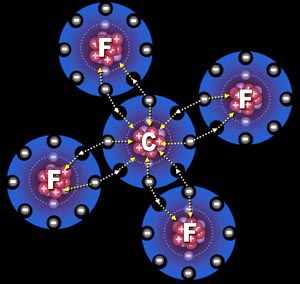
Chemical Bonds: The Atom Connection This tutorial reviews some bonding principles from an earlier tutorial that focused on building blocks and expands it to focus on force & energy plus a more modern view of the atom. Quiz on Bonds at Sapling Learning |
 |
|
|
Composition, Misconceptions, Gases at Work, Gas Laws, and Density |
 |
|
|
Quiz on Gases at Sapling Learning |
 |
|
|
Liquids, Solutions, Concentrations Tutorial Quiz on Liquids, Solutions, and Concentrations at Sapling Learning |
 |
|
|
Acids
and Bases
|
 |
|
|
See Sapling Learning or Canvas for information on the course final and departmental final In the image, that's not a zero and no, that's not CHM130 students collapsed on the grass. Take a breath, the "O" stands for oxygen. |
 |
|
Last Updated 9/18/18 9:14 AM