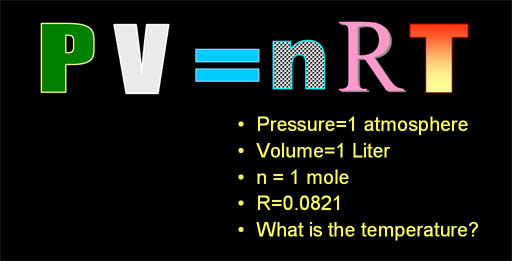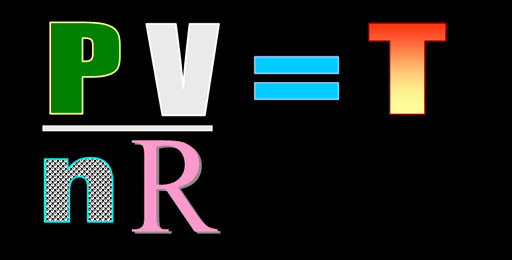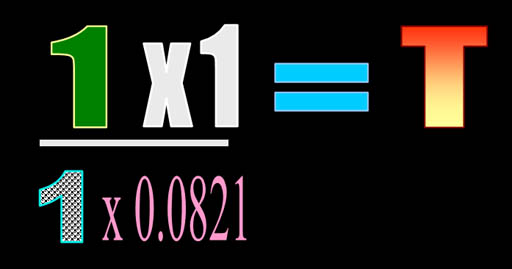|
Composition
of Clean Air
|
|
|
|
In the fall of 2002 I got to go to Switzerland.
On the morning of November 6th, I had to leave the village of Zermatt,
but wanted to take one more look at the Matterhorn. The air was absolutely
clear. Cleaner than I have ever seen air anywhere. The Matterhorn jumped
out at you. By the way, Zermatt does not allow automobiles. People walk
or ride electric vehicles. The train that goes there and the trolley that
takes you to the ski lifts also run off of electricity.
|
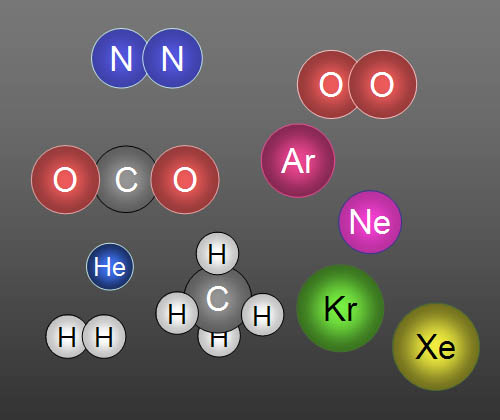 |
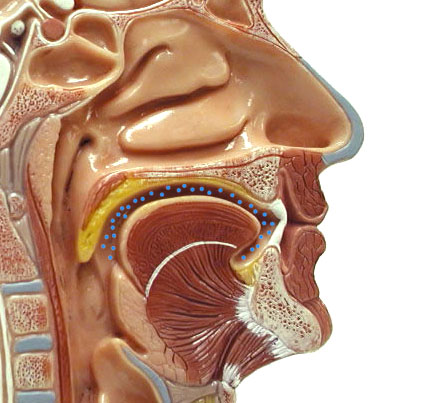
Here are 10 gases that make up clean air: In
order of highest to lowest concentration they are Nitrogen, Oxygen, Argon,
Carbon dioxide, Neon, Helium, Methane (CH4), Krypton, Hydrogen,
and Xenon. Five of them travel alone, so we call them atoms.
For example, a helium balloon contains atoms
of helium (He), but an oxygen tank contains molecules
of oxygen (O2). When there's two or more atoms are bonded together,
they are called molecules. |
|
|
NITROGEN
(N2) #1: 8 out of 10 atoms
(or molecules) of air are made of nitrogen. Nitrogen gas can't be seen
but when it is cooled to -320 °F (-195°C) it turns to a liquid,
which you can see. Being this cold makes it handy for many things. Shown
here, it is being used to quickly freeze cream and sugar to make ice cream.
|

|
NITROGEN #2:
Liquid nitrogen is also used to cool certain metals to the point where they
become superconductors. Superconductors conduct electricity (electrons)
without any resistance. In this picture a rectangular shaped magnet had
been dropped onto a metal disk. As the magnet fell toward the metal disk,
the magnetism in the magnet caused currents of electricity to flow inside
the metal disk. The currents created in the metal disk by the falling magnet
made that part of the metal disk a magnet itself. The falling magnet then
feels the repulsion of the magnet it just created. The falling magnet will
then stop just above the metal disk. Electricity continues to flow in the
metal disk constantly creating a mirror image magnet that repels the real
magnet. This will continue forever as long as the metal is cool enough to
stay a superconductor. |
|
|
NITROGEN #3: Oxygen reacts
with many things including paint. Therefore, expensive works of art and
other rare relics are stored in pure nitrogen. Nitrogen is fairly inert
and doesn't react (combine) with most other substances, so it is used
to protect items and keep oxygen away..
|
|
|
|
NITROGEN goes bad: Under
high temperatures, like in a jet engine or car engine, nitrogen will combine
with oxygen to form a class of toxic
compounds called nitrogen oxides. The simplest having one nitrogen and
one oxygen (NO). Others have two nitrogens and one oxygen (N2O),
one nitrogen and two oxygens (NO2), and the fourth has two
of each (N2O2). Their names (in order) are nitrogen
oxide, dinitrogen oxide, nitrogen dioxide, and dinitrogen dioxide. Nitrogen
dioxide has a brownish appearance and is often what you see in polluted
cities.
|
 |
Oxygen
#1: 2 out of 10 atoms/molecules of air
is oxygen. Oxygen is produced by plants and is consumed by animals. Animals
need oxygen to "burn" the food they eat in order to get the calories
(energy) they need. The way animals use oxygen to burn food is different
than a fire, but it produces the same products of carbon dioxide and water.
Of all of the gases that make up air, oxygen is the most reactive. |
|
|
|
OXYGEN #2: Oxygen
is everywhere. As a gas it makes up 20% of the air. Some oxygen gas is
dissolved in water, and some oxygen gas is in the pores in the ground.
However, there's a lot more oxygen in this picture that we don't
normally consider. The weight of the water in the river is 90% oxygen
atoms. Remember water is H20. The one oxygen in H20
weighs 8 times has much as the two hydrogen atoms combined. The
trees and grass are made of carbon, oxygen, hydrogen, and nitrogen, with
oxygen accounting for about half of a plant's weight. The rocks,
the soil, and the mountains contain metals and silicon that is bound to
oxygen, which makes up about 1/2 of their weight. Think about it; one
half of a mountain's weight is from oxygen. The only thing in the picture
that doesn't contain oxygen are the metal rails.
|
|
|
| OXYGEN #3:
In the movie Total Recall, there was an ancient alien factory that could
release the oxygen from the Martian soil. This is actually possible. Extreme
heat will cause the oxygen to separate from the metals and non-metals that
it was bound to. Most of Martian soil is iron oxide, which would turn to
iron metal and oxygen gas at high temperatures. In the pictures above, Arnold
presses the touch sensitive on "button" of the oxygen generator.
Next he gets blown out of the factory into the thin Martian air, which
causes the air in his body to expand. Fortunately, the oxygen rushes out
of the top of the mountain that housed the factory and they show Mars getting
an atmosphere similar to Earth's. Actually, in the movie, they say the alien
factory is getting oxygen from water, which is possible but a lot of hydrogen
would be also created, making an explosive mixture. My idea of getting it
from the red iron oxide would be safer. |
|
|
ARGON:
Argon is 1 out of 100 atoms/molecules of air. Argon is an inert gas that
is used to pressurize light bulbs. Being inert it doesn't react with the
tungsten metal, which makes the filament. The argon gas also helps keeps
the tungsten metal atoms on the surface of the filament from jumping off
the filament. |
|
|
CARBON DIOXIDE:
Carbon dioxide only makes 3 out of 10,000 atoms/molecules of air; however,
it supplies the carbon that plants use to make leaves, trunks, roots, etc.
It's hard to believe that the weight of giant redwood trees came from the
small amount of carbon dioxide gas in the air. |
 |
NEON:
2 out 100,000 atoms/molecules of air. Neon is a clear inert gas; however,
high voltage can strip electrons off neon atoms. As the electrons fall back
into place, they give off ultraviolet light (black light), which you can't
see. However, the glass tubes are coated with different phosphor powders,
which glow when ultraviolet light hits them. |
|
|
HELIUM:
5 out of 1,000,000 atoms or molecules of air are helium. Helium is the second
lightest gas (hydrogen is lightest). It is used to fill toy balloons and
weather balloons. It is so inert that there is no danger of igniting nor
will it combine with other elements. For this reason it is used in welding
by having helium gas surround an electric arc that is melting a metal. The
flow of helium gas keeps the oxygen away from the metal so that the oxygen
won't oxidize (rust) the metals being welded. |
|
|
| METHANE:
2 out of 1,000,000 atoms/molecules of air are methane molecules. Bacteria
is a big source of methane gas and this type of bacteria is found in termites
(see huge termite mound), cattle (cow flatulence), rice paddies, swamps
(called swamp or marsh gas), and in the sea bed. In the sea bed, methane
can be found trapped in ice (see person holding ice that's on fire). Methane
also comes from petroleum fields and is the natural gas you cook with. An
embarrassing source of methane is from the eating of beans. |
 |
KRYPTON:
1 out of 1,000,000 atoms/molecules of air is krypton gas. You may remember
krypton as the name of the planet that Superman came from. The pieces of
the planet were called kryptonite. The element krypton is used in lamps
in a similar way that argon is used (see above). The word krypton comes
from "Krypto" meaning hidden. The gas krypton was in a way hidden
because it took quite a while for scientists to discover it. |
 |
HYDROGEN:
1 out of 2,000,000 atoms/molecules of air is hydrogen. Hydrogen is the lightest
element, which is why is was the preferred gas to fill blimps and dirigibles.
Unfortunately, hydrogen is very flammable, which led to the catastrophic
tragedy of Germany's Hindenburg airship. |
|
|
XENON:
87 atoms out of 1,000,000,000 (one billion) atoms/molecules of air are xenon.
Like argon and krypton, xenon is used as the gas in lamps. This one is for
a car's headlamp. These are probably the ones that look more blue. |
|
|
| WATER VAPOR:
The other gas we didn't list before is water vapor. The concentration of
the other gases are pretty consistent, but water vapor varies greatly. Using
a fish-eye lens, I took this picture of a double rainbow in Geneva, Switzerland.
It had rained all day, but just before the sun went down, the clouds parted
enough to allow the sunshine to strike the droplets of liquid water still
in the air. Water in vapor form (gas) is not visible. |
 |
One Last Look
at Composition of Air: Imagine the volume of
air in a typical classroom that is 30 feet by 30 feet with a 10 foot high
ceiling. Also assume, we separated all the gases. Oxygen would cover the
room to about 2 feet deep. Nitrogen would fill almost to the ceiling (another
8 feet minus a couple of inches). Argon gas would fill a one inch layer
over the whole room. The remaining gases fill the last one inch. Carbon
dioxide has about the same volume of one student. Neon is 1.5 gallons. Helium
would fill a one liter bottle. Methane gas would fill someone's 1/2 liter
bottle. Krypton would fill a 12 oz soda can. Hydrogen would fill about half
of a 12 oz soda can. And xenon gas would have the volume of a pencil's eraser.
|
|
Misconception
#1: There's a lot of air
|
|
|
|
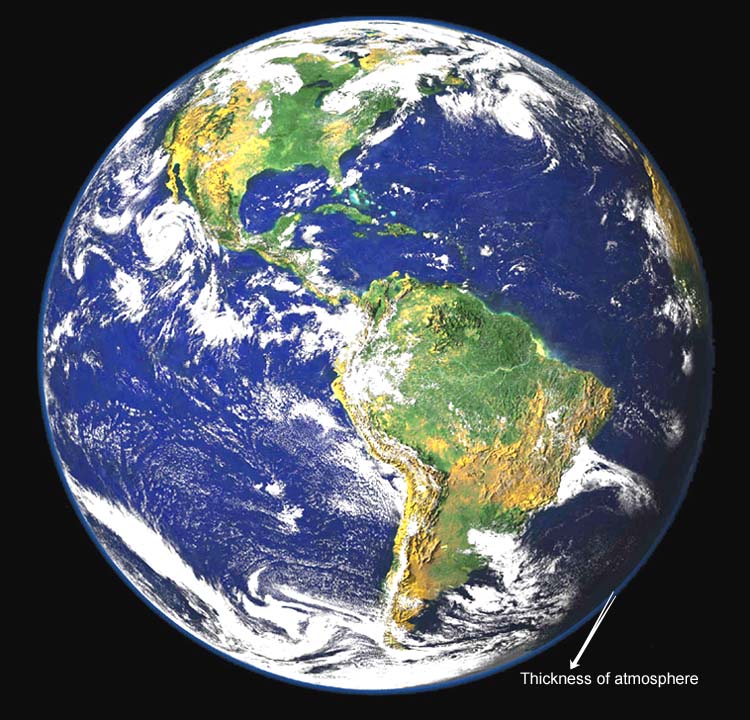
To us the atmosphere seems very thick, but compared to
the Earth, it is only a thin skin of gas. Seeing how thin it should help
us realize how easily it could be filled with pollutants.
Besides giving us the oxygen we need to breathe, it also
shields us from harmful ultraviolet radiation and small meteors. The atmosphere
also helps hold in the warmth of the sun and spreads the warmth more evenly
over the Earth's surface. It also carries water from the oceans
to the land by way of clouds and rain.
|
|
Misconception #2: Air
is light weight
|
|
|
Misconception #2: Air is light weight.
We don't feel the weight of air nor do balloons seem heavy.
It is true that for the same volume, air is lighter than liquid
or solids. But there are many miles of air above us pushing down
with incredible weight.
|
|
|
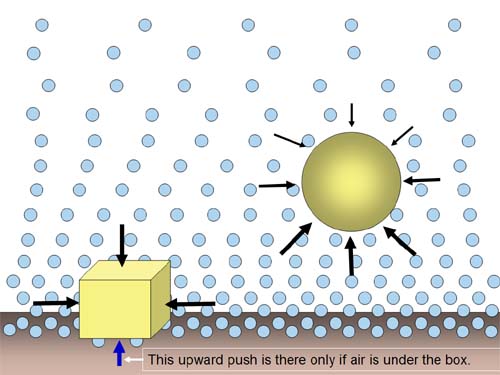
| Air is so heavy that it can lift this Jet.
Even when the jet is sitting on the ground, the air underneath the jet is
lifting with several thousand pounds of force. However, the air on top of
the jet and on top of the wings is pushing it down with practically the
same thousands of pounds of force. |
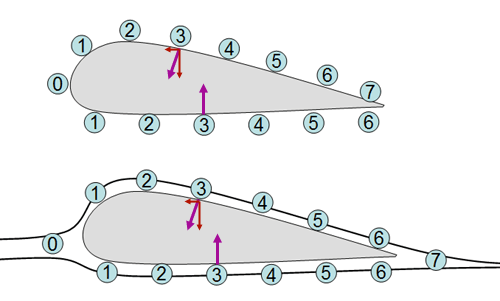 |
This is how the plane can fly. The top
drawing shows the wing not moving in the air. Because the top of the wing
is curved, there is more surface area and more air hitting it. The 7 balls
represent the number of air molecules hitting it. Underneath the wing
there are only 6. The top 7 don't push down more than the bottom 6 because
the 7 are hitting at an angle which decreases their downward push. For
example, the top #3 molecule has the same force (pressure) as the bottom
#3, but the top #3 hits at an angle reducing it's downward force.
But when the wing is moving, the air splits
at 0 and rejoins at 7. So the top doesn't have 7 hitting it anymore, just
6. The six on the top of the curved wing hit at angles which reduces their
downward force. The 6 under the wing hit the wing with a push that goes
straight up. So the bottom 6 out-push the top 6 and the plane is lifted
up.
|
|
|
Air gets heavier as it cools. For example,
sometimes rain falling in a thundercloud cools the air fast and the heavy
air comes crashing downward. The dropping speed is usually about 45 miles
per hour but can reach 200 miles per hour endangering planes, people, and
property. This is called a microburst. |
|
|
Normally the air gets cooler as we go higher,
but sometimes the air near the ground is colder than the air above it. Because
cold air is heavier, it will stay close to the ground. This traps pollutants.
This condition is common in Phoenix in the winter and results in the infamous
brown cloud. |
|
Misconception #3: Calm
Air
|
|
|
Since air is invisible it's easy to think of it just being
still because we've heard the phrases, "calm air" and "the
air was still". However, even when air is not blowing, it is far
from being still.
|
|
|
In a helium balloon, the helium atoms are traveling an
average of 3,000 miles per hour! In air, the oxygen and nitrogen molecules*
are traveling about 1,000 miles per hour! At these incredible speeds,
one atom will collide with other atoms and objects near them 7 billion
times a second!
(*Note: Oxygen atoms travel in pairs as does nitrogen
atoms. Two or more atoms combined is called molecules. So we say a gas
of oxygen molecules and nitrogen molecules rather than saying oxygen atoms
and nitrogen atoms)
|
|
|
|
|
This means that if a small air
leak develops in the vacuum of space, air molecules rush out over a 1,000
miles per hour. There is no atmosphere to hold back the leak. |
|
Misconception #4: Suction
|
|
|
Suction Cups:
The way suction cups work is usually very much misunderstood.
Contrary to popular believe, suction is not
what holds suction cups to the surface.
|
|
|
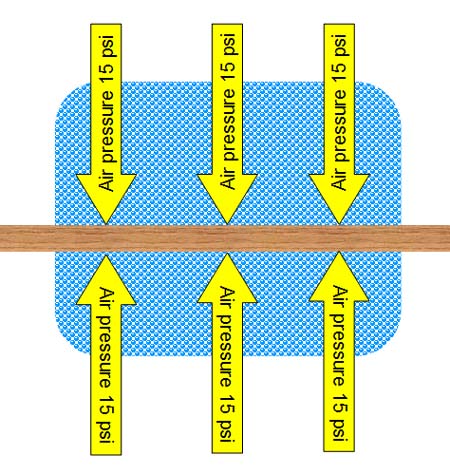
Before a suction cup even attaches to a surface,
there is air pressure pushing on the surface at about 15 pounds per square. |
|
|
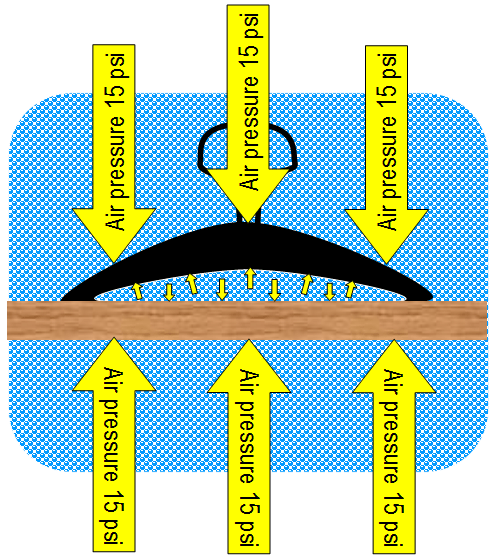
When the suction cup touches the surface, air
is still between the suction cup and the surface (e.g., table top or window).
Air underneath the suction cup presses in all directions, which includes
pushing up at 15 psi. The suction cup can easily
be pulled away at this point. |
|
|
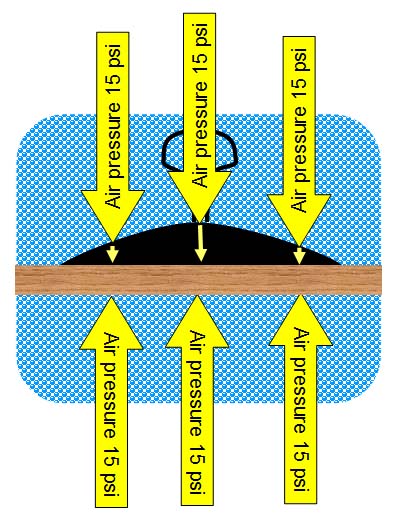
However, if the suction cup is pressed all the way down
to expel all of the air, there is no longer any pressure pushing upward
on the suction cup and the outside air pressure is now pressing down on
the suction cup. This is what holds the suction cup in place.
So there is no sticking or
attraction force that makes the suction cup stick to the surface. It is
held there by air pressure. It just seems
that the suction cup has some unseen stickiness holding it there.
|
|
|
Note: In a similar fashion, any time air gets
squeezed out from between two objects, they will be held together by air
pressure. For example, if you've ever walked through mud, you noticed how
hard it is to lift your feet. That's not because mud is sticky or thick.
It's because air gets squeezed out from between your shoes and the mud.
Air pressure will try to hold your feet and shoes down. |
|
|
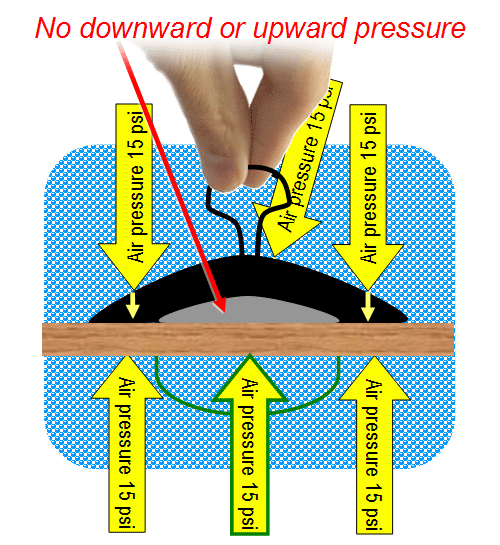
Sometimes suction cups are used for lifting or pulling.
Let's say the surface is that of a table. When a person pulls on the middle
of a suction cup, they have to overcome the pressure of the 80 miles of
air above the suction cup, which is 15 pounds per square inch. When they
do, the suction cup lifts a little creating a vacuum gap. A vacuum is
nothing, so it has no force. So it no longer pushes down on the surface.
However, underneath that surface, air pressure is still pushing up at
15 psi, so it will lift the table (provided the table doesn't weigh too
much). For example, if the vacuum gap covers 3 square inches, then 3 square
inches under the table will push with 45 lbs (3x15 psi.) To lift a heavier
table, you need a bigger suction cup that can make a vacuum gap with a
bigger area.
|
|
Drinking through a Straw
|
|
|
Even though babies know how
to drink from a straw, most people, young or old, don't
know how it works. Most people think the suction
caused by our mouth pulls the liquid
up through the straw. |
|
|
One clue to understanding this is to notice what happens
to the cheeks of people drinking through a straw. This is especially noticeable
if the drink is thick like a shake. You see that their cheeks are pushed
in.
This is caused by the air pressure outside their cheeks
being higher than the air pressure in their mouth.
|
|
|
Normally when your mouth is closed, there's
not much air (blue spheres) inside your mouth. They bounce around causing
15 psi pressure in the mouth. However, when you drop your jaw and keep your
lips closed, there's more room for the air to spread out (Roll
mouse over image to see this). The air molecules are now spread out
over a larger volume, so fewer are now striking each square inch of the
inside of the mouth; so the air pressure inside the mouth is less (perhaps
about 10 psi). Outside air at 15 psi is trying to get into the mouth. It
pushes on the cheeks causing them to be sunken. |
|
|
If you have a straw in your mouth, then air pressure pushing
on your drink has more force than the force
from the air in your mouth. The outside air pressure pushes onto the surface
of the drink. This pressure pushes liquid
up through the straw to your mouth. When you don't want to drink anymore,
you will move your jaw upwards causing the
air in the mouth to be more crowed, which increases the air pressure in
the mouth to equal that of the outside air pressure. The liquid will stop
flowing.
|
|
|
To the left is a microscopic image
of globules of fat in milk. When it was first seen through a microscope,
they though these spheres were living microbes because they kept moving.
Later they realized that there were simply being knocked around by something
invisible, which was water molecules in motion. (roll
cursor to see short clip of the motion). This same jostling motion
is seen with dust or smoke particles. They are getting knocked around by
molecules of air. |
|
|
Here I've made a one dimensional
animation of a dust particle getting bounced back and forth. (Roll cursor
over image). Remember each air molecule has about 7,000,000,000 collisions
per second. Since the collisions are equal on both sides, the particle
stays almost in the same location. Now imagine if a fan were to push one
of the air molecules on the right side away from this region. What happens
is the moving air molecules on the right side have fewer air molecules to
bounce off of, so they would travel farther to the right before bouncing
back. The collisions on the left side would keep on coming at their same
rate. Therefore, the dust particle is going to be pushed to the right. This
is how a vacuum cleaner works. Air is blown out of the yellow container
shown, so there's less air in the container. Less air means less collisions.
However, outside the number of air molecule collisions have not gone down,
so particles are going to get knocked into the vacuum cleaner by the outside
air. We should also credit gravity for keeping the outside air compressed.
|
|
Misconceptions #4 & 5: Air
is uniform & Hot Air Rises
|
|
|
Air is indivisible, so when we
look at things through the air, we normally think the air is uniform. If
that were true, this balloon would not float. |
|
|
The air around us is being compressed
by all of the air above us. The closer the air is to the ground the more
weight is on it, and the more compressed (or dense) it is. That means
the air at the top of an object is not as dense as the air under objects.
In fact the air around us is constantly trying to lift everything because
the air below objects press a bit harder than the air above the object.
|
|
|
In this picture, I replaced some
of the balloons with bowling balls to make the point that air is pushing
up on bowling balls or helium filled balloons the same amount. (as long
as they are the same size). Again, that's because the air under a bowling
ball or balloon is more dense (compressed) than the air near the top of
the bowling ball or balloon. |
|
|
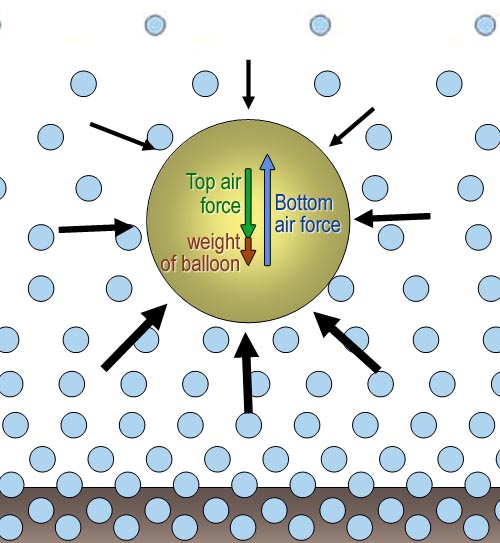
The balloon rises because the
air pushing on the bottom of the balloon has a greater force than the downward
force of the air on top of the balloon plus the balloon's own weight. In
the case of the bowling ball, the red weight arrow is so large, the upward
lift from the bottom air goes unnoticed. |
|
|
So the real reason hot air balloons float,
is NOT "Hot Air Rises." It's because the
air pressure on the lower half of the balloon is greater than (the
combined air pressure on the upper half plus
the weight of the balloon). All the hot air
does is to reduce the weight
of the air in the balloon. Hot air is more spread out than cool air,
so it's lighter. When the burner is on, the air inside is heated and as
it expands much of it goes out the bottom of the balloon. This reduces the
red arrow, which represents the total weight of the balloon. |
|
PUTTING GASES
TO WORK
|
|
|
These high tech
jet fighters take off from an aircraft carrier with a boost from one of
the oldest of technologies — steam. |
|
|
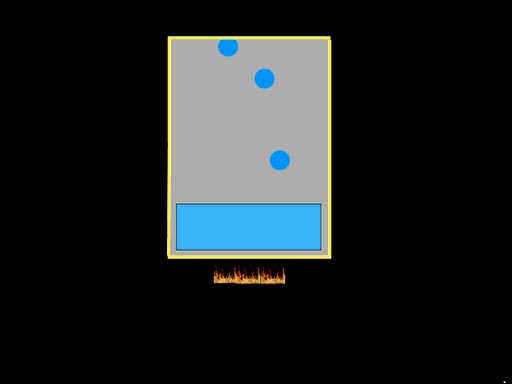
The secret to water and steam
power is that the pressure inside a closed container will get very high
when water is heated. That's because more and more water molecules will
go into a gas phase, which dramatically increases the pressure. Normally,
the pressure is used to push a piston like in the jet fighter launch above
or in a steam engine. If the pressure isn't released, the container is likely
to explode (roll cursor over image for animation). |
|
|
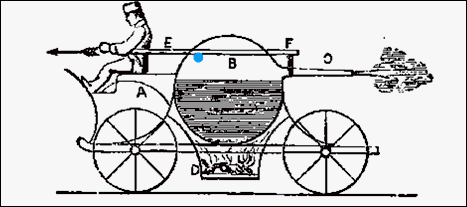
The very first engine was powered
by steam. It was invented by Hero of Alexandria, Egypt. Water was placed
in a sphere and then heated to boiling. Small tubes allowed the steam to
release. The openings caused an imbalance of the pressure in the sphere,
which spun the sphere. A similar engine is done with a thermos bottle filled
with liquid nitrogen. As the liquid nitrogen warms and turns to gas, the
escaping gas causes the thermos to spin. |
 |
A common misconception is that exhaust, whether it be
steam or flames, is the reason for the thrust and the propulsion. It looks
that way because that's where all the action is. However, how can flames
outside the missile actually do any pushing on the missile? Usually people
think that the flames are pushing on the air and that gives the missile
propulsion. But missiles work just as well in space where there's nothing
to push on.
|
|
|
To see what really gives the missile thrust,
let's look at a car that was designed to use steam propulsion. Whenever
there's pressure built up inside a chamber due to steam or exploding gases,
the pressure is pushing on in all directions and all
sides of the chamber. The chamber (or vehicle) doesn't move because
pressure is equal in all directions. But if
you have an opening on one side of the chamber, the fast moving gas molecules
causing the pressure have nothing to bounce off of (there's a hole there).
That means the the pressure on the side opposite
of the opening is not getting canceled
by any pressure at the exhaust opening. So the chamber gets pushed by gas
molecules hitting the chamber opposite of the opening (toward the front).
(roll cursor over steam car to see animation).
Realize it's the collisions of gases opposite
of the exhaust opening that pushes the vehicle
or missile. |
|
|
Using steam for propulsion like
jet propulsion isn't very efficient. The better way was have the steam pressure
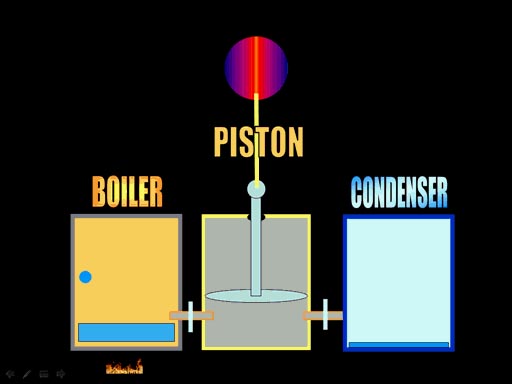
push on a piston. Here are the basic components of the steam engine. First
there's the boiler, which is where you heat water and get a lot of pressure
built up. Next you open a valve to the piston cylinder, which let's in the
high pressure from the boiler. That pushes the piston up. At the top of
the stroke, you open a value to the condenser, which is cool and condenses
the water so it has no pressure. Outside air pressure will push the piston
down because there's very little pressure left in the piston's cylinder.
(Roll cursor over image to see animation) |
|
|
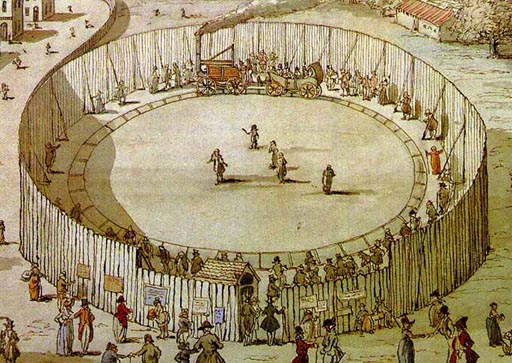
In the early days of steam engines,
they were such a novelty that people were charged admission to come look
at a steam engine powered train. |
 |
Steam engines were quite the work
horse in factories and on the farm. Anything that could burn could be used
to turn water into steam. Once you had steam pressure you could get it to
push a piston that would then turn a wheel. The large wheel (pulley) on
top was fitted with a large leather belt. The belt could be used to spin
another pulley that was attached to a saw, a pump, or whatever the factory
or farm needed to power. It was quite versatile. The drawback was all the
smoke from whatever fuel was used to heat the water. A new engine that had
much less smoke exhaust was the gasoline engine. |
 |
Gasoline Engine: The word "gas" is an alteration
of Latin, chaos. It's well chosen because when gas burns, the molecules
are in chaos.
Here's an article from 1897 talking about the new gasoline
engine.
"The gas engine is one of
the wonders of the 19th century. Now, within three years of the 20th century,
it is a novel machine, eagerly sought by many people. It is thought by
persons who have not studied its principles that it is a steam-engine,
using gas or gasoline as fuel for the purpose of making steam. This is
erroneous. Gas and gasoline in specific proportion with air are explosive
material."
"The expansive force derived
from explosion of these materials in the cylinder is the force that is
substituted for the expansive force of steam. Hence, owing to the economy
of this method as a means of deriving power, the steam engine and boiler
are fast disappearing, and the Gas Engine is taking their place for small
power."
|
|
|
Even though the gasoline engine
created much less pollution than did the steam engine, there's still a lot
of pollution when there's so many cars. Here's Phoenix with its famous brown
cloud caused by nitrogen oxides coming from car exhausts. |
|
Gases that Obey the Law
|
|
|
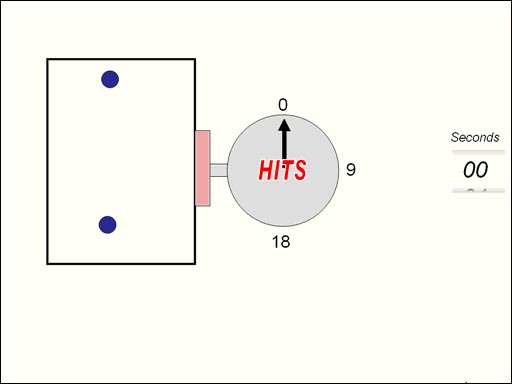
There's several laws that gases
obey, and I honestly don't remember what name goes to what behavior. It's
more important that one understands the basic behavior of gas. One of the
laws has to do with pressure versus volume. The molecules of gas are bouncing
around inside this box. There's a sensor that counts the hits. The hits
are proportional to the pressure. Next the box will get half its size and
the hits are counted again. What you see if that the hits are doubled if
the volume is halved. (Roll cursor over image for
animation). |
|
|
This law is actually quite logical.
If the volume decreases, the pressure increases
because as the volume (size) of the box gets smaller the molecules of gas
don't have to travel as far to bounce into each other or the sides of the
box. So they bounce into each other and the sides more often causing increased
pressure. |
|
|
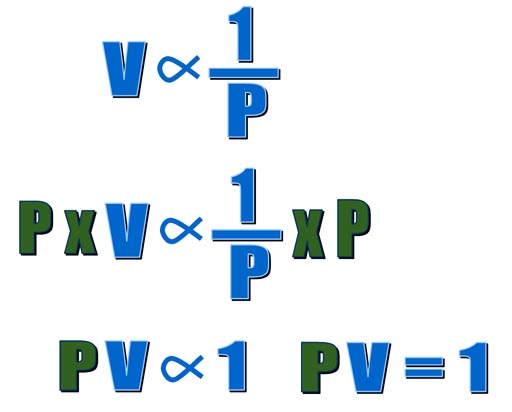
When one value goes up and another
goes down, they call that inversely proportional. In math they write it
as shown on the left. This says volume is inversely proportional to pressure.
If we multiply both sides by pressure (P), we get another equation that
also says pressure and volume are inversely proportional to each other. |
|
|
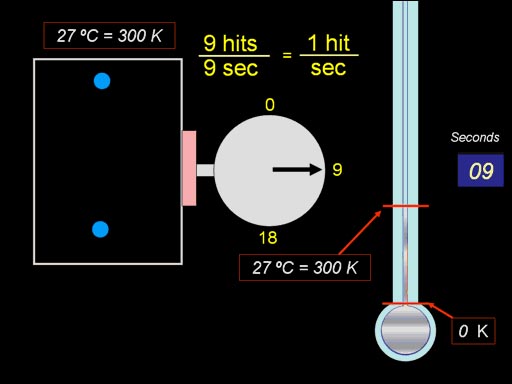
Another law for gases involves
pressure versus temperature. There's a name for it, but again, I don't remember.
However, this animation will reveal the concept. First the pressure is measured
at 81°F (27°C or 300K). We get 1 hit per second, which is an indication
of pressure. Now we heat up the gas in the box to double the temperature.
We count the hits again, and it has gone up to 2 hits per second, which
means the pressure doubled. (Roll cursor over image
for animation). |
 |
The above animation shows that pressure is directly proportional
to temperature. In other words, if temperature goes up, pressure also
goes up. If temperature goes down, pressure must go down.
When this is combined with the knowledge that pressure
is inversely proportional to volume, we get the bottom relationship. That
says the product of pressure multiplied by volume is directly proportional
to the temperature.
|
|
|
Earlier I had the animation that
showed liquid water becoming water vapor with these extra water molecules
increasing the pressure. This demonstrates that pressure is directly proportional
to the number of gas molecules. (Roll cursor over
image for animation). |
|
|
Our formula is now factoring in
the influence of the number of gas atoms or molecules that is contained
in the volume. In many cases the gas is held in a container that can't stretch,
so when more molecules are added, the pressure has got to increase. (Roll
cursor over image for animation). |
|
|
In this animation, I will again
reinforce the idea that when volume goes down the pressure will increase,
and then the opposite, which is when the volume can get larger and large,
the pressure will go very low. Again, this makes sense because the collisions
of the gases with themselves and the sides will get less and less as the
volume increases (roll cursor over image for animation) |
|
|
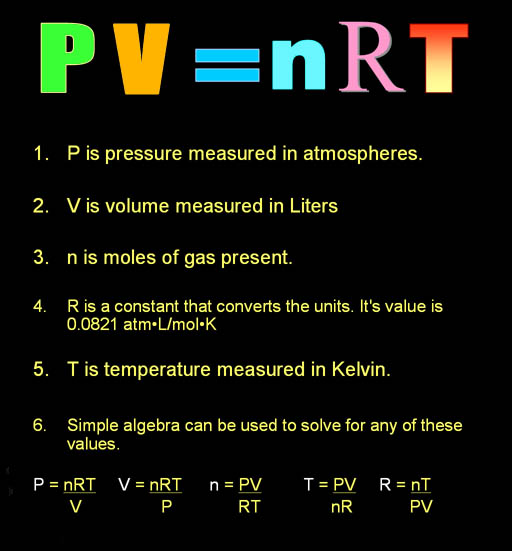
By using a conversion factor,
"R", we can set these values equal
to each other. This law has a name I remember. It's called the "Ideal
Gas Law" and explains how an ideal
gas behaves. This means a gas who atoms or molecules don't stick
to each other. They just bounce off like balls.
In this formula, we will measure pressure (P)
in atmospheres (atm),
which is the pressure at sea level. Volume
(V) should be in liters
(L) . "n" represents the
count of the gas atoms or molecules in moles
(mol). "R"
is a constant that converts these units. To
do that it has a value of 0.0821 atm•L/mol•K.
"K" is the temperature
measured in degrees of Kelvin.
Simple algebra can be used to solve for each of these values. That way you
can solve for pressure, volume,
moles, or temperature
by knowing the other units. "R" is a constant so it doesn't really
need to be solved. |
|
|
Car talk is an entertaining radio
show that talks about car issues, but these car experts have a lot of chemistry
under their belt. One caller had a problem with the pistons that hold up
the hatchback to their car. To explain it, they mention the ideal gas law.
Click on link to hear this 3 minute dialog.
CarTalkAudioClip.mp3 |
|
|
Let's do a problem using the ideal
gas law equation. The question is we have a gas inside a one liter volume
and the pressure is 1 atmosphere. There's one mole of gas in the one liter,
so what must be the temperature? It must be cold because in earlier tutorials,
I said one mole of gas is 22.4 liters. However, I was assuming it was at
one atmosphere of pressure (14.7 psi=pounds
per sq. inch)
and 0°C. Here the pressure is the same, but it must be much colder for
the volume to shrink from 22.4 liters down to just one liter. |
|
|
To solve for temperature, we divide
both sides by nR. Then we plug in the values. |
|
|
Now we have 1 atmosphere for pressure,
1 liter for pressure, 1 mole for quantity, and the 0.0821 conversion constant.
Normally, I'd write all of the units, but for now let's keep it uncluttered.
So the math is pretty easy. Multiply 1 times 1 and divide that by 1 x 0.0821.
The answer turns out to be 12.2 K . That's close to absolute zero. That's
-261°C or -478°F. Like I said,
we expected it to be cold because the gas is in a volume over 22 times smaller
than normal. |
|
|
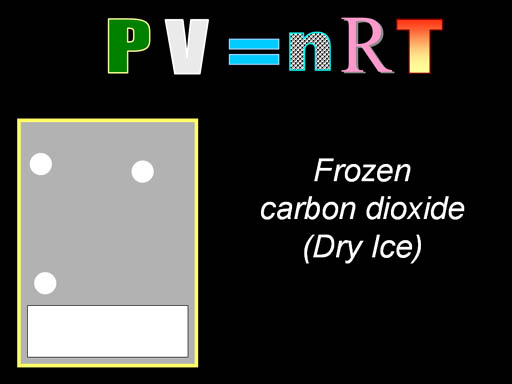
Let's do another problem that
has to do with dry ice evaporating inside a close container. As the dry
ice changes to vapor, pressure will increase dramatically inside of the
container. This technique is used to make dry ice bombs out of 2 liter soda
bottles. Fortunately, the bottle is plastic so the plastic shrapnel isn't
too dangerous, but the bottle cap can do harm. (Roll
cursor over image if you want to see an injury to the forehead when a bottle
cap blew off. Warning: it's a bit graphic).
That guy probably will think twice about doing that again. |
|
|
Here's the question: What
pressure could be reached when ¼ lb of dry ice is placed in this
2 liter bottle? Temperature is 86
°F (30 °C).
Since pressure is asked
for, the ideal gas law is solved for P. Now
we see that we need moles (n), temperature
(T), and volume. The problem says it's
a 2 liter container. It doesn't give moles,
but we can convert pounds to grams,
and then grams to moles.
The temperature of 30°C
has to be convert to Kelvin by adding 273.
That gives us 303K. When we plug those 3 values
into our formula, we get the answer of 32.3 atmospheres. If we want the
answer is psi, just multiply by 14.7 psi/ATM, which gives us 475 psi. That's
a lot of pressure. Most air compressor tanks only go up to 120 psi. A heavy
duty car tire will blow out at around 70 psi. So this bottle is definitely
going to blow up.
|
|
|
So far we mentioned pressure in atmospheres and in pounds
per square inch (psi). I'm sure you've heard of other units. The easiest
way to measure air pressure was to pour mercury into a tube that was about
a yard tall. Then put the thumb on the opening and turn it upside down
with the opening submerged in a bowl of mercury. You would think gravity
would just pull the mercury down; however, air pressure in the bowl pushes
on the surface of the mercury in the bowl which then pushes in all directions
including upward to hold the mercury up. At sea level the air pressure
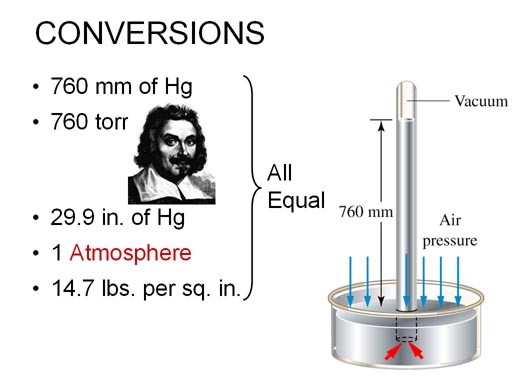
holds the mercury 760 millimeters (29.9) inches above the surface of the
mercury in the bowl.
Instead of 760 mm, some people call it 760 torr
after a scientist named Evangelista Torricelli.
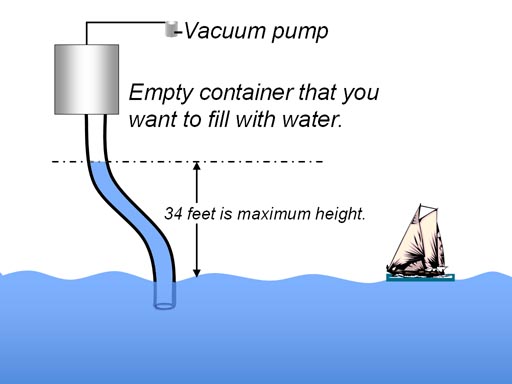
If water is used in the tube, air pressure can hold a
column of water that is 34 feet high no matter how large of a diameter.
|
|
|
By hooking a vacuum pump to a
container and pumping out the air (making a vacuum) you can get air pressure
to push the liquid up into the container. Remember a vacuum cannot "suck"
things into it. The lifting force only comes from the difference in air
pressure. The more air pumped out of the container, the bigger the difference.
Even if all air is pumped out, water will only rise 34 feet because at 34
feet water weighs 14.7 lbs. per sq. inch equaling the air pressure. |
|
|
For those of you going into a
medical field, you will undoubtedly see a device for measure blood pressure,
which is measured in millimeters of mercury. One of the more expense types
actually uses mercury in a tube and the height of the mercury is how you
read the pressure. These devices are called either sphygmomanometers or
sphygometers. |
|
Gas Density
|
 |
Gas Density is important to emergency responders because
they need to know if the toxic gas is going to be low to the ground, up
near the ceiling, or mixed with the air. Knowing the density tells them
that.
I've mentioned that a lot of these non-metal form compounds
that are toxic. It's smart to know if they are heavier or lighter than
air. The Periodic Table will help you calculate the density.
|
 |
Air is mostly N2. So the grams
per mole is 14.01 x 2=28g. Since O2 is a little heavier, the density of
air is actually 28.8 grams per mole. Let's see if fluorine gas sinks or
rises in the air. Fluorine (F2) is 38.0 grams per mole (19.0 x 2). So it
will lay close to the ground, which means if there's a fluorine leak, run
out of the area on your tiptoes. Cl2 is 35.45x2=70.9g/mole, so it is very
heavy. HF is (1.008+19=20.0). It will rise and be near the ceiling. So to
escape, crawl out of the room. All of these toxic gases can be done the
same way. |
| Congratulations on getting through this long
tutorial. The quiz, I promise, will be a lot shorter. |