
Reading:
11th: pages 83-96
12th: pages 84-98
13th: pages 81-93
14th: pages 82-93
It's a short chapter, so read the whole chapter. My tutorial will reinforce a lot of what you read; however, there are some extra information in the textbook.
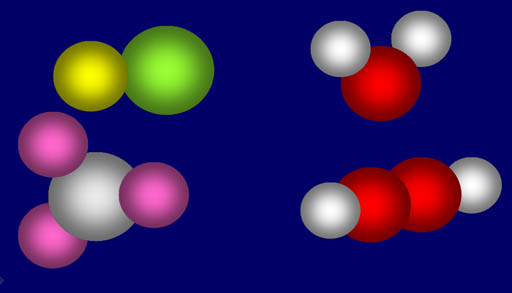

Table 5.1: 11th-page
86, 12th-page 87, 13th-page 84, not in 14th edition.
The textbook shows a
table of the percent composition of various compounds. Unfortunately,
it made the same mistake as in a previous chapter of not saying what kind
of percent. Remember a compound is made up of two or more elements. Se
each element makes up only a fraction of the whole compound. For example,
the upper right molecule is water (H2O). If the percent is
by number then hydrogen is 67% because it is two out of three atoms. If
the percent is by weight, then hydrogen is 11% because the hydrogen atoms' weight is
only 11% of the whole molecule's weight. The table shown in the textbook
is percent composition by weight. The reason percent by weight is used
is that the early methods for analyzing compounds was to break the individual
elements away from the compound using heat or chemicals. Then weighing
what was left or the new compounds formed. This was called an assay. A
common assay was to determine the percent mass of silver or gold in an ore.