Boiling water freezes before hitting the ground.
Some sections of this chapter are not in the CHM151 competencies, so we will be selective with the reading.
In all versions of the textbook, read the following sections:
10.1 Intermolecular Forces
10.2 The Liquid State
10.3 An Introduction to Structures and Types of Solids
10.8 Vapor Pressure and Changes of State
10.9 Phase Diagrams
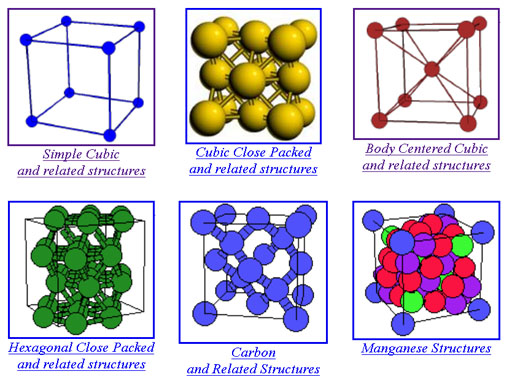
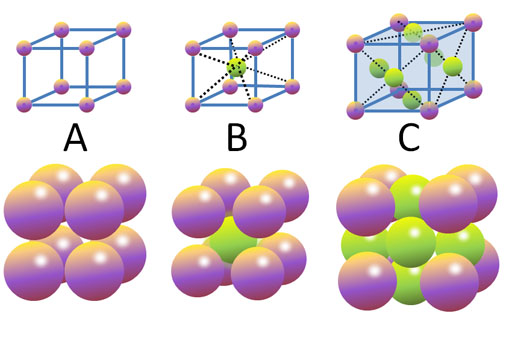
Here is one site that has pictures and links to 3-D visualization of the crystals. The first six types are the most common ones. Click on them to go to that page that has more examples.
http://cst-www.nrl.navy.mil/lattice/
If you click on the Simple Cubic structure shown above, it takes you to a page that has more examples of that structure. If you click on one of these crystal types, it opens a page that lets you see it from various angles. There is also a link called "visualize the structure". It uses the JMOL Java applet, which you might already have installed on your computer. If so, you can view it in 3-D.
Watch out for all of the new terminology. Realize that's more than you need to get into right now. Just get familiar with the basic shapes.
Chapter 10 Textbook Problems
You don't need to look them up in the textbook because I've reproduced the problems below.
Since I didn't do a tutorial for this chapter, I will give some mini-tutorials as you do the problems below

Problem 1: What is stronger, intermolecular or intramolecular forces for a given molecule? What observation(s) have you made to support this?
Mini-tutorial: In the last few chapters, the focus was on the bonds between atoms in a molecule. There were ionic bonds and covalent bonds discussed. Those are the "intramolecular forces" they are talking about. Then there are the forces between the molecules which hold the molecules together as a gas, liquid, or solid. Those are the
"intermolecular" forces. Even without knowing what are these intermolecular forces, we can answer which is stronger. Boiling water is one observation. By heating liquid water on the stove we can cause the water molecules to come apart and turn into steam. So we overcame the intermolecular forces of water molecules with some heat. However, we know that in the steam, it is still water (H2O). The bonds between hydrogen and oxygen were not broken. So the bonds within the molecules (intramolecular forces) must be stronger. When we melt or vaporize something, we overcome the intermolecular forces. If that substance does not decompose, then the intermolecular forces are weaker than the intramolecular forces (bonds). So give an example of another substance that you melted or boiled that didn't decompose.
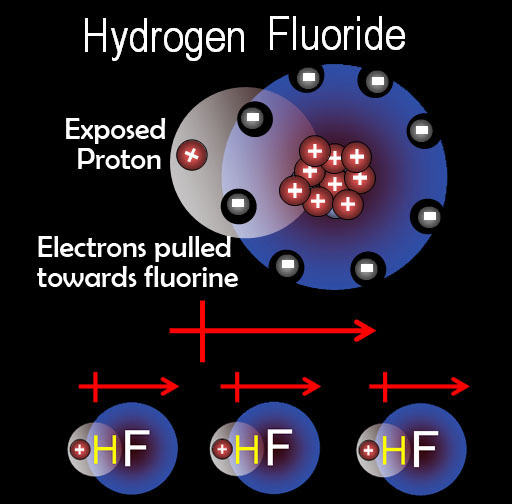
Problem 2: Identify the most important types of interparticle forces present in the solids of each of the following substance.
| a. Ar | b. HCl | c. HF | d. CaCl2 |
| e. CH4 | f. CO | g. NaNO3 |
Mini-tutorial: The textbook switched words. Here they said "interparticle" forces instead of "intermolecular" forces. The only reason is that argon (Ar) is not a molecule, but the meaning is the same. All 3 intermolecular forces are electrical forces.
Dipole forces: The word dipole literally means "2 poles". That's redundant because a pole has to have 2 ends otherwise it's not a pole. So the "di" is more for emphasis. There are "2" ends. One is positive and the other is negative. On the left is HF. Fluorine's 9 protons pulls harder on hydrogen's electron so there's more negative charge around fluorine. Hydrogen's proton is partially exposed making the hydrogen partially positive. That forms a dipole (represented by the arrow with the + end. What that means for intermolecular forces is that this +/- dipole causes attraction between HF molecules. As always + is attracted to -.
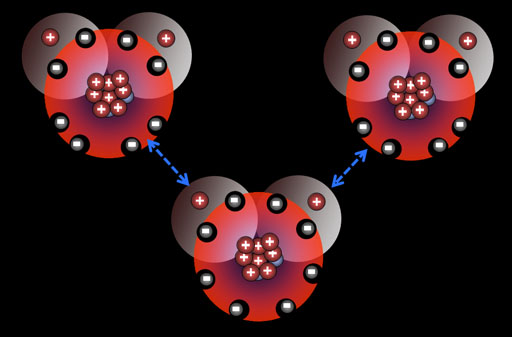
Dipole forces (Hydrogen bonding): Water molecules are similar to HF because they also have an imbalance of charges. The oxygen end is more negative than the hydrogen side. That means there's dipole (+/-) attraction. Also, since these hydrogen atoms have had much of their electron cloud pulled away, the hydrogen's nucleus (a proton) is partially exposed. This proton is attracted to any lone pairs on the oxygen atom on the neighboring water (blue arrows). They call this hydrogen bonding, and it's fairly strong, which is why water is a liquid at room temperature. That also explains why water has to be heated so much to boil.
Note: The dipoles within HF and H2O are permanent. The third force is a temporary dipole. It's called either Van der Waals force or London Dispersion force.
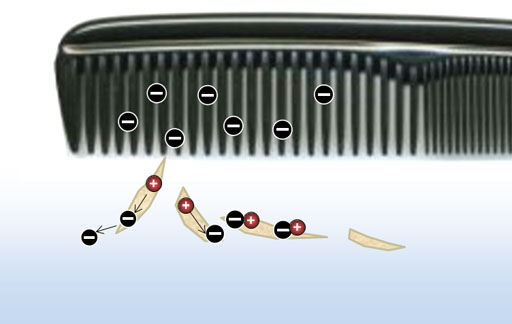
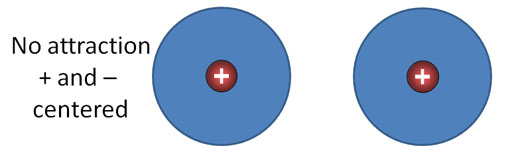
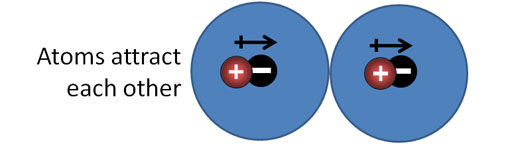
London Dispersion forces (continued): Look at the blue sphere as an electron cloud that surrounds a positive nucleus. There are two atoms side by side. Their positive and negative charges are centered, so there's no net attraction or repulsion.


Problem 2 (repeated): Identify the most important types of interparticle forces present in the solids of each of the following substance. (See some hints below)
| a. Ar | b. HCl | c. HF | d. CaCl2 |
| e. CH4 | f. CO | g. NaNO3 |
(Hint: 2a. Argon atoms are have its charge centered like the example above. However, at cold temperatures, the atoms can bump each other causing their electron cloud and nucleus not to be centered. That will cause a dipole which will influence other argon atoms if they are going slow enough (extreme cold).
2b. HCl like HF explained above.
2c. Attraction force for HF was explained above.
2d. CaCl2. Calcium has a +2 charge and chlorine atoms have a minus 1 charge each. So these form dipoles (+ and - charges) The charges in HF and HCl are only partially negative and partially positive charges. They are not as strong as the ionic compounds like CaCl2. So the intermolecular (interparticle) forces are high due to strong dipole attractions.
2e. CH4. In methane the carbon and hydrogen atoms have equal pull on their electrons (equal electronegativity). So there are no dipoles formed. So there's little attraction between methane molecules. The only attraction force is the temporary London Dispersion forces that occur if the electron cloud of one the hydrogen atoms gets pushed off center.
2f. CO has a dipole because oxygen has a much greater pull on the shared electrons than does carbon. So the oxygen end is partially negative and carbon's end is more positive. So this + and - charge attracts other CO molecules using dipole attraction.
2g. NaNO3. These are ions (Na+ and NO3-). So they are like 2d.
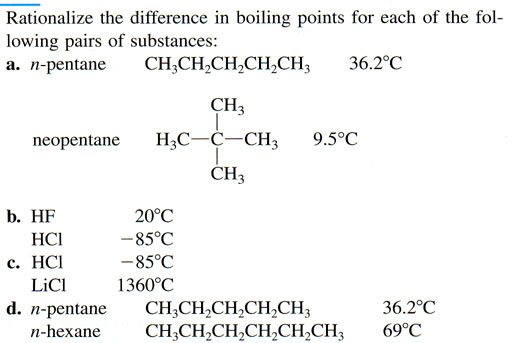
Problem 3: On the left is the problem. Here I will give some tips.
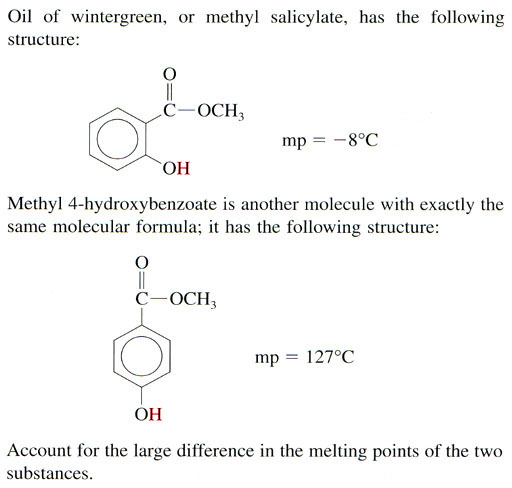
a. n-pentane has a higher boiling point than neopentane. So that means n-pentane must have stronger intermolecular forces. Remember the London Dispersion force above. If an atom that has a dipole can get close to another atom, it can induce a dipole in that other atom, which will cause them to attract each other. The shape of n-pentane allows those molecules to stack on each other, which gets atoms closer to each other. Do you think neopentane molecules can stack up on each other as easily?
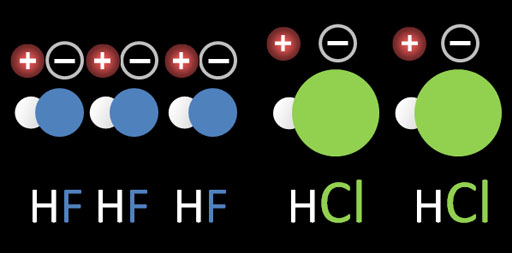
b. HF has a much higher boiling point than HCl meaning HF has more attraction between the HF molecules. Chemically they are both very similar. The difference is that Cl is quite a bit bigger than F. See image on the left. Because fluorine is smaller, the + and - separations are closer, which makes the attraction stronger. The larger chlorine atom spreads out the + and - charges, which makes the attraction weaker.
c. This is a dramatic difference. Lithium is a full + charge because it lost its electron to chlorine, which is a full negative charge. In HCl, the hydrogen atom is only partially charged and the chlorine atom is only partially negatively charged.
d. n-hexane has a higher boiling point, so that means it has a stronger attraction between other n-hexane molecules. I think you can figure out why.

(Hint: There are some misconceptions about boiling water. We think heat is the only reason water boils. We forget that air pressure is just as important. In a vacuum, water boils at room temperature, so it doesn't take any extra heat to make it boil. So less air pressure means it will boil at a lower temperature. The reverse is true too. Pressure cookers cook faster because they keep more pressure in the pot, which causes the water to boil at a higher temperature helping the food to cook faster.)


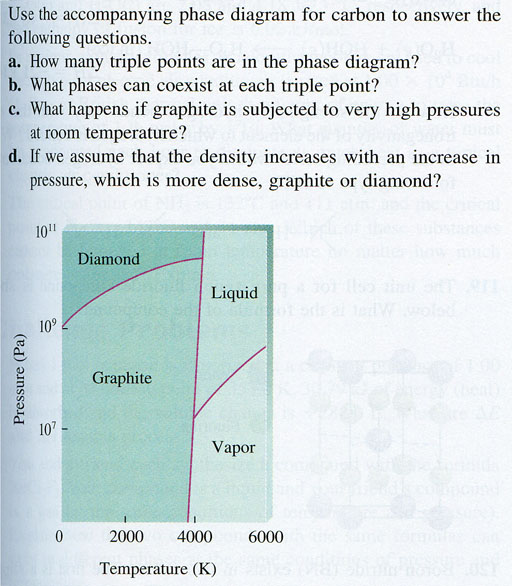
Diamonds are not forever
Problem 6: When wet laundry is hung on a clothesline on a cold winter day, it will freeze but eventually dry. Explain.
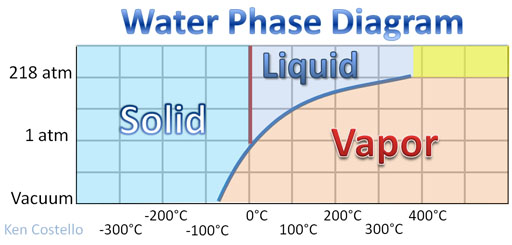
Mini-tutorial: Freeze dried food is fairly common. I always liked the freeze-dried ice cream. These foods were not frozen and hung on a clothesline, because that would take too long. The food is put in a vacuum to speed up drying. Why would that help? The lack of pressure allows the water molecules to easily break free. How does water turn into vapor if it is frozen? This is another misconception about temperature. When a thermometer is used to measure the temperature of something, it is only getting the average temperature. In other words it is only measuring the average kinetic energy (energy of movement) of the atoms or molecules. So even if ice has a temperature of, let's say, -5°C and nearly all water molecules are locked up in ice crystals, there are a few of the water molecules moving much faster than the average movement and are capable of breaking loose from the other frozen water molecules. So the ice does evaporate. Matter of fact in a vacuum at even a chilling -50°C, ice cannot exist in a vacuum. See Water Phase Diagram. Only water vapor can exist at that pressure and temperature.
I also read an article about how chemical reactions can occur in ice if given enough time. A scientist mixed some simple compounds and kept them frozen for 20 years. The big surprise was these basic building blocks produced many complicated compounds such as amino acids and short proteins. Some of the success is from the fact that even if something is frozen, some atoms and molecules get momentarily much hotter.
In the textbook, there's a similar interesting story about how diamonds will slowly turn to graphite. Again, a few carbon atoms at a time, that are locked up in the diamond crystal, will gain the energy necessary to break the strong tetrahedral sp3 bonds and form triagonal sp2 bonds that graphite uses.
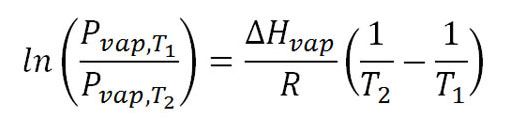

Problem 7: In Breckenridge, Colorado the typical atmospheric pressure is 520. torr. What is the boiling point of water in Breckenridge, Colorado?
(Hint: Torr is the same as mm Hg (millimeters of mercury) and is named after Evangelista Torricelli. The textbook gives a formula that lets you solve for vapor pressure or temperature depending on what you have. In this case we are given air pressure of 520 torr. But that's not the vapor pressure of water; however, when water boils, its vapor pressure has to equal that of the air pressure. So that means we know the vapor pressure is 520 torr at boiling. So 520 is water's vapor pressure at the boiling temperature in Breckenridge. In the formula we can replace Pvap,T1with 520. T1 is the unknown boiling point temperature we are looking for. Like all algebra equations, we can solve for the one unknown if we know the other values. This formula works if we know another temperature (T2) and the vapor pressure at that temperature (Pvap,T2). The problem did not provide that but we know that at 100°C water boils which means it matches the air pressure of 760 torr (760 mm Hg or 1 atm). So Pvap,T2 is 760 torr and the T2 is 100°C.
The textbook does give the heat of vaporization ΔHvap or we can look it up. That is given as 40.7 kJ/mole (That's 40,700 joules per mole or per 18 grams). R is a constant and is 8.3145 J/K·mole. Those units remind us that the energy needs to be in joules (J) and temperature needs to be in Kelvin (K). So the 100°C needs to become 273+100 = 373K. Also, the 40.7 kJ (kilojoules) needs to be written as 40,700 joules. Then our units will cancel.
In the equation "ln" stands for natural log. That's different than the base 10 log used in pH. Log 100=2 because 102=100. So 10 is the base. "ln 100=4.6" because the base is 2.71828. So 2.71824.6=100. In the second equation the variables have been replaced with their values. The next step is to simplify the values. In a spreadsheet I used "=ln(520/760) to get that value. The values are simplified and then the formula is solved for T1. You can see why you have algebra before CHM151. I took it up to 373=T1+0.028917T1. You can solve for T1. Then remember that is in Kelvin. Turn that in Celsius. You will find a temperature lower than 100°C. That's why cooking by boiling requires more time at these higher altitudes.
Problem 8: An ice cube tray contains enough water at 22.0°C to make 18 ice cubes that each have a mass of 30.0 g. The tray is placed in a freezer that uses CF2Cl2 as a refrigerant. The heat of vaporization of CF2Cl2 is 158 J/g. What mass of CF2Cl2 must be vaporized to convert all the water that begins at 22.0°C (72°F) to ice at -5°C? The heat capacities for H2O(s) and H2O(l) are 2.03 J/g·°C and 4.18 J/g·°C respectively, and the enthalpy of fusion for ice is 6.02 kJ/mol (6,020 J/mol).
(Help: You can design refrigerators with theses kind of calculations. This problem is similar to those in the thermochemistry chapter except we must have the heat (enthalpy) of vaporization and heat (enthalpy) of fusion as sources of heat.
It's clear that the heat that the water gives up to turn to ice will have to be absorbed by the refrigerant going from liquid to gas (heat of vaporization).
The cooling of water happens in 3 stages. It first goes from 22.0°C down to 0°C, then it has to cool more as it freezes. Finally, the ice needs to cool from 0°C to -5°C. All of those joules of energy can be added up. Once that is known, the mass of refrigerant is not hard to find. Begin by finding the mass of water that needs to be frozen: 30.0 g per cube x 18 cubes = 540 g. The heat of fusion of water (heat from freezing) is given in joules per mole, so we have to convert the grams to moles.
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
|
| 1 | Mass of water |
Degrees cooled |
Heat capacity of water |
Energy lost |
||||||
| 2 | Energy lost to cool water to 0°C | 540 |
g | 22.0 |
°C | 4.18 |
J | = |
??? |
Joules |
| 3 | g·°C | |||||||||
| 4 | ||||||||||
| 5 | Energy lost as water becomes ice (no temperature change) | Mass of water |
Convert g to moles |
Heat of fusion of water |
Energy lost |
|||||
| 6 | 540 |
g | 1 |
mole | 6020 |
J | = |
??? |
Joules | |
| 7 | 18 |
g | mole | |||||||
| 8 | ||||||||||
| 9 | Energy lost as ice cools to -5°C | Mass of water |
Degrees cooled |
Heat capacity of ice |
Energy lost |
|||||
| 10 | 540 |
g | 5.0 |
°C | 2.03 |
J | = |
??? |
Joules | |
| 11 | g·°C | |||||||||
| 12 | Total Joules | = |
??? |
Joules | ||||||
| 13 | Use the total joules and the heat of vaporization of CF2Cl2 (158 J/g) to find grams of refrigerant needed. |
|||||||||
To download the above spreadsheet (Excel format), click here.