In the Building Block Tutorial Series, one of the tutorials covered the basics of chemical bonding. We will review some of those concepts and also update it using concepts from the Modern Atom tutorial.
Atoms are made from protons, neutrons, and electrons. Since bonding involves electrical attraction, it's the protons and electrons that are more important.
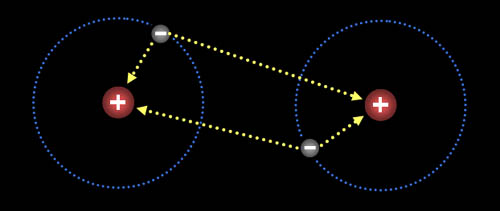
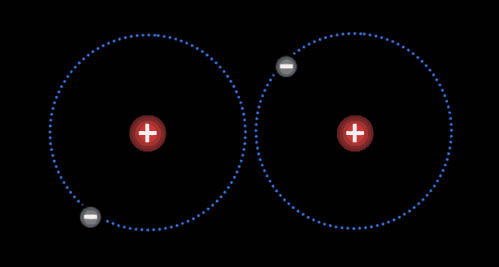
Here are two hydrogen atoms. Notice that the electron on the left atom is attracted to both its own proton and to the right proton of the right hydrogen. Likewise, the electron in the right hydrogen is attracted to both protons. So there's a balance of repulsion and attraction. With no attraction, atoms would never make a bond. With no repulsion, the atoms would just merge together as one atom. So balancing attraction and repulsion allows for atoms to form compounds.
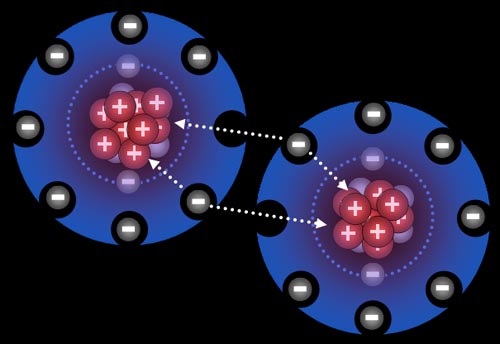
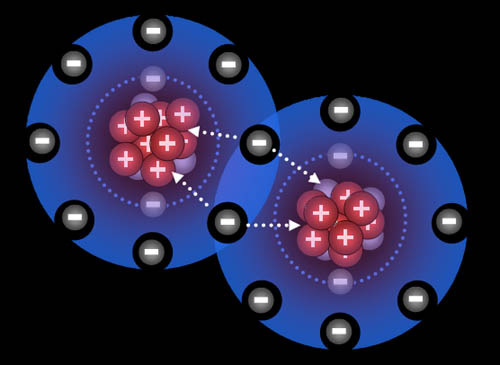
Here the fluorine atoms have pulled together and are sharing one electron each. They are now bonded. This is called a single bond (even though two electrons are involved) Because they pull on these shared electrons equally, they call this a covalent bond. "co" meaning "shared" and "valent" meaning "strength". So the strength of the bond is shared equally.
Atoms naturally pull on each other because the protons in them pull on the electrons of the other atoms. However, there has to be room for the electrons to merge and be shared by both atoms, otherwise electron repulsion keeps them apart.
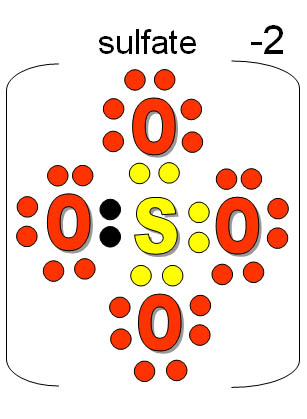
Note: Most non-metal atoms have room for eight outer electrons.
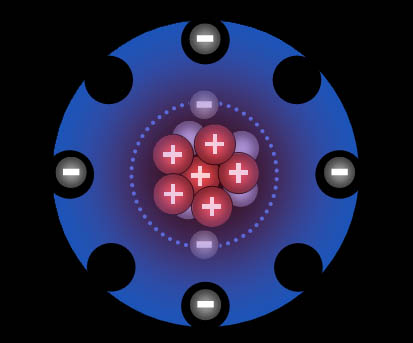
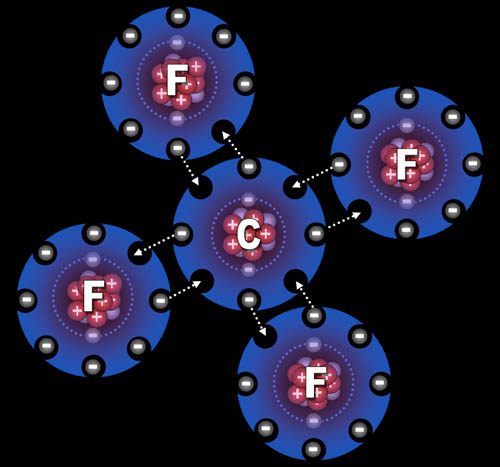
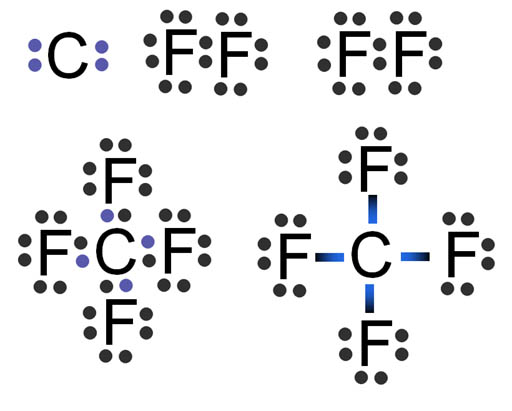
Fluorine "wants" one electron to fill up its outer shell of electrons. Carbon will provide that electron as long as fluorine shares one of its electrons with carbon because carbon also wants to attain 8 outer electrons (8 is called an octet).
You can see how four fluorine atoms can connect to one carbon atom. They come together to form carbon tetrafluoride, which is a gas used in refrigeration systems. With nine protons, fluorine's pull on that electron is stronger than carbon's pull because carbon only has 6 protons. This uneven sharing of the electrons means fluorine is slightly negative because the electrons are pulled closer to it. Carbon is partially positive because its outer electrons are closer to the fluorine atoms and not canceling out its own positive charge. For this reason, they call this a polar covalent bond. "Covalent" because the electrons are shared and "polar" because there's a + and - charge separation (like the ends of a pole). Roll cursor over the image to see the + and - charged poles.
The electron can change shape in order to maximize space between it and other electrons. Electrons are always moving and their exact position is not set but based on probability. In other words, the orbits we've been showing are their most probable position. But there is a chance that electrons can be elsewhere at least for a moment. There's probably several electrons in your body that may somewhere else in the room at this moment. Again, electrons are amazing little "particles."
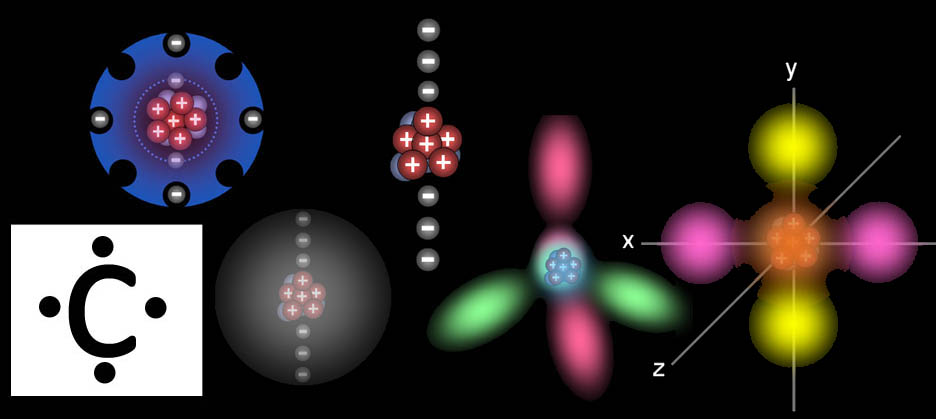
Try clicking on the above link to play a Quicktime movie. It will likely launch Quicktime. In the movie above, you see carbon with its six electrons orbiting it. (If that doesn't work, try this link: Play Quicktime movie). Again, this is a simplistic view of the electrons. First, let's change the inner two electrons to the spherical electron cloud that better represents them (the 1s orbital). The next two electrons the 2s orbital. The next two electrons, however, form the shape of four lobes with each electron being two lobes. Those are the 2px and 2pyorbitals. Sometimes when carbon bonds with other atoms, the two 2s electrons and the two 2p electrons will blend their shapes to form four equally shaped lobes that project out in four directions. The arrangement of the lobes match a four sided pyramid called a tetrahedron. A tetrahedron allows the electrons maximum separation in 3 dimensions. More about this in the next tutorial.
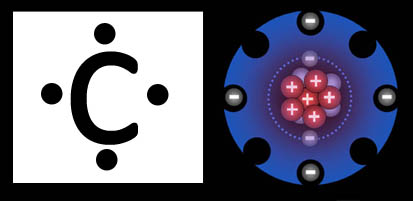
Lewis Dot Structure
vs.
Costello Hole-Punch Structure

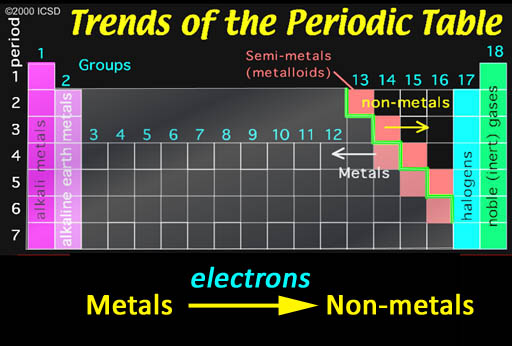
Ionic Bonding: Who bonds with whom? The non-metals in the upper right have a strong pull on electrons and, in contrast, metals tend to let go of their electrons easily. So when they get together, you can guess what happens. It's like putting a greedy person together with a generous person. You can guarantee money will leave the generous person and end up owned by the greedy person. The same thing is true about non-metals and metals. In contact with each other, the metals are usually giving away one or more of their electrons to the non-metals. When this happens, the metals become positive ions because they lost one or more the negative electrons. The non-metals become negative ions because they gained electrons. The metal and non-metal will attract each other because they have opposite charges. That's ionic bonding.

The exception are the noble (inert) gases. They already have 8 outer electrons, which is a very stable configuration. So they won't share any electrons and therefore won't bond with other elements.
The halogens (group 17) are one electron short of a stable 8 configuration (called an octet). That makes them the most "greedy" of all elements. In contrast the metals in groups 1 & 2 are the metals that give up their 1 or 2 electrons the easiest.
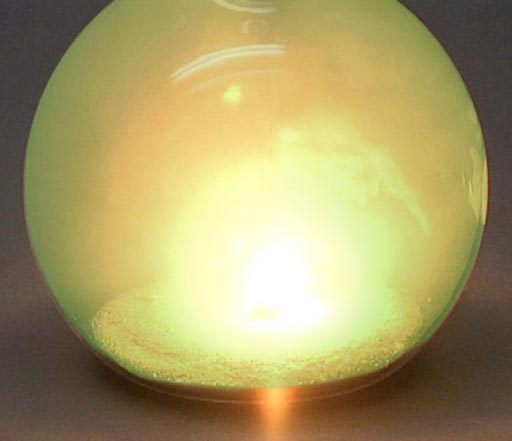
So when the halogens come in contact with the metals from the first two groups, step back because there's going to be a violent reaction as electrons transfer from these metals to the halogen elements.
Here chlorine gas is reacting with sodium metal. After all the fireworks, all that's left is NaCl that you can place on your French fries (assuming you have equal numbers of chlorine and sodium atoms present).
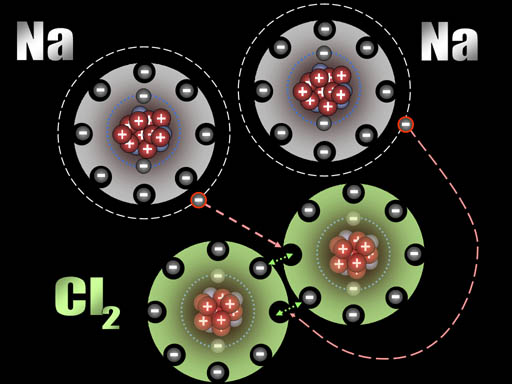
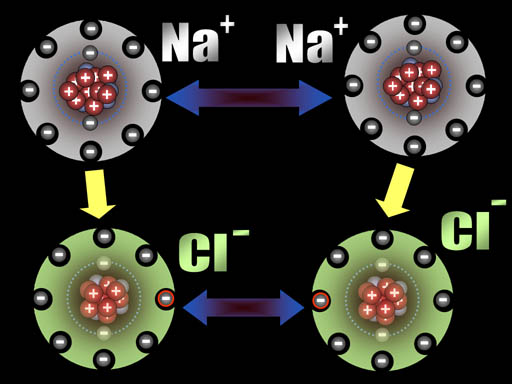
After those two electrons have transferred, the sodium atoms are now positively charged (+1) because the protons in the nucleus out number the electrons in the atom by one. The chlorine atoms are now negatively charged (-1) because each gained an extra electron.
All atoms are now ions because they have a charge. The two sodium ions will push away from each other because they are both positive. The two chloride ions will push away from each other because both are negative. However, since sodium and chlorine are now oppositely charged, they will be attracted to each other. That attraction is a bond appropriately called an ionic bond.

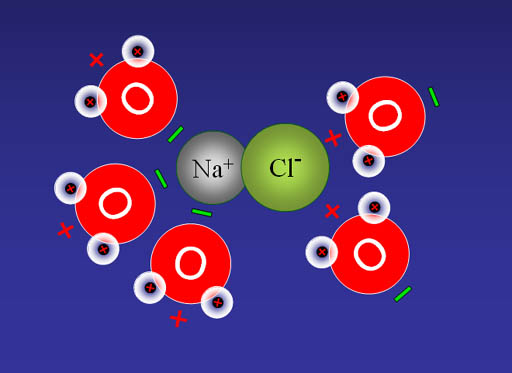
Na+ and Cl- are both very stable because they have the 8 outer electron configuration (octet). That's why our world has trillions of tons of salt (NaCl).
So what does this octet look like?
In the earlier tutorial on Types of Chemical Reactions, we focused on the building block aspect of reactions. In this tutorial on bonds, we focus on the force & energy aspect of bonds involved in these reactions.
Types of Chemical Reactions
Synthesis (Combination)
Decomposition
Single Replacement/Displacement
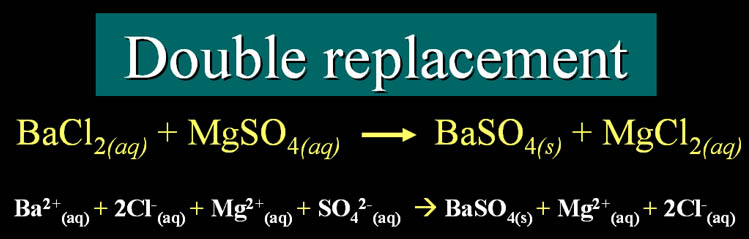
Double Replacement/Displacement
Oxidation (Combustion)

2Al + 3O2 ->
2Al2O3
Aluminum reacts with oxygen to make aluminum oxide (ingredient of sapphires). Since
aluminum loses all 3 of its outer electrons to oxygen, it forms a strong ionic
bond.

Synthesis: Inorganic compounds
Consider a metal with a non-metal. They form ionic bonds because electron(s) will
leave the metal and be captured by the non-metal. That will make them oppositely
charged, which then draws them together. In Al2O3, the ions are Al3+, Al3+, O2-, O2-, and O2-. As these ions come together they release energy. The same thing happens for any opposite charges.
O2-Al3+O2-Al3+O2-
Mg2+O2-
Na+Cl-
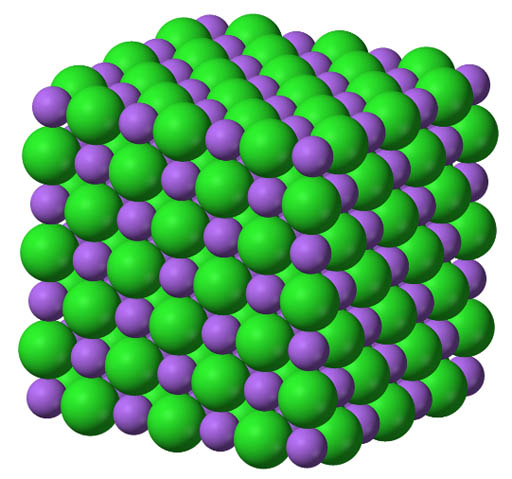
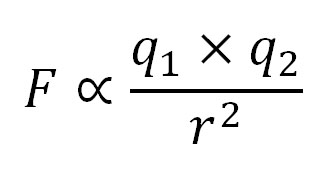
On the left I show 3 examples of compounds that have ionic bonds.
When the positive and negative ions come together, they don't just form a single molecule. They form a crystal (or lattice) of stacked + and - ions. The energy released as they come together to form this lattice (crystal) is called lattice energy. Here is the lattice energy for the 3 compounds listed. The negative sign (-) means it releases energy.
Al2O3 is -15,916 kJ/mole /5 atoms≈3183 per atom
MgO is -3,795 kJ/mole / 2 atoms≈1898 per atom
NaCl is -787 kJ/mole / 2 atoms≈394per atom
You can see that Al2O3 releases the most energy, which makes it quite stable. That's one reason why it is so hard and makes good gemstones.
I divided by the number of atoms to see the average energy released per atom. Al2O3 releases the most per atom. Why? For electrical attraction, two things matter. One is the amount of charge involved and the other is the distance between. On the the left, the equation says electrical force is proportional to the charge (q1) times the charge (q2) divided by the distance between them squared (r2).The spreadsheet below breaks this down. You will see that the Al3+O2- bond has the highest charge and the distance between them is theclosest. Combined, that makes the calculated force between them 12.8 times stronger than that between Na+ and Cl- in NaCl.
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
|
| 1 | + charge |
- charge |
+ × - |
Radius of + ion |
Radius of - ion |
r = Distance between charges (ion radii added) |
Force + × - r2 |
Relative forces |
Actual lattice energy per atom ratios |
|||||
| 2 | Al3+ |
3 | O2- |
2 | 6 |
Al3+ |
0.535 |
O2- |
1.4 |
1.935 |
1.600 | 12.8 |
8.1 |
|
| 3 | Mg2+ |
2 | O2- |
2 | 4 |
Mg2+ |
0.72 |
O2- |
1.4 |
2.12 |
0.888 | 7.1 |
4.8 |
|
| 4 | Na+ |
1 | Cl- |
1 | 1 |
Na+ |
1.02 |
Cl- |
1.81 |
2.83 |
0.125 | 1 |
1 |
|

Synthesis: Organic compounds
All organic compounds have carbon in them. As covered earlier, carbon
has 4 outer electrons, so it can share these 4 while accepting four. The
bonds are covalent bonds since it's a sharing of the electrons. More specifically
it's a polar covalent bond because, as mentioned before, the fluorines
have a much stronger pull on the shared electrons than does carbon. The
molecule, as you might guess, is carbon tetrafluoride.
The fluorine bonds are stronger (lower energy) than oxygen bonds, which is why this compound won't burn. Oxygen can't break the fluorine-carbon bond.


Decomposition: For decomposition reactions it's nice to have compounds whose bonds can break fairly easily. This is the ammonium dichromate decomposition reaction shown in the Type of Reactions tutorial. Regarding bonds, you can tell from the products which bonds are the strongest. Apparently N2, H2O, and Cr2O3 have stronger bonds. Water is a product which means the hydrogens came off of the ammonium (NH4) and the oxygens came off of dichromate (Cr2O7). You could deduce that the bonds in water (oxygen to hydrogen) are stronger than the nitrogen to hydrogen bonds in ammonium (NH4) and some of the oxygen to chromium bonds in the dichromate (Cr2O7).
Also, since 3/4 of the world's surface is water, we could say the bonds in water must be strong (stable and lower energy). So a lot reactions produce water because once hydrogen and oxygen come together to make water, it's hard to break those bonds.
Single Replacement/Displacement
This is the example reaction given in
the Types of Reaction tutorial.
Zn + FeCl2(aq) ->
Fe + ZnCl2(aq)
The (aq) means it's dissolved in water. That's always a clue that it's
an ionic bond. Also because it's a metal combined with a non-metal, that's
an ionic bond because the metal loses its electron(s) to the non-metal.
Ionic bonds are usually quite strong. For example, you can heat FeCl2
up to 1300F and it will melt but not decompose. However, put a few drops
of water on it at room temperature, and the bonds will break. So ionic
bonds are interesting. They are very resistant to heat but many will be
easily broken by everyday water.

Oxidation (Combustion)
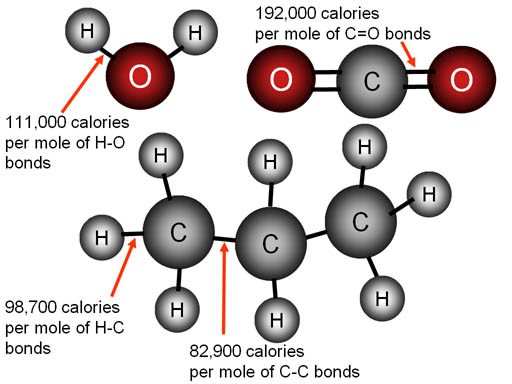
This is where bond strength makes combustion possible. The 3-carbon hydrocarbon
is propane. When it burns, bonds between the hydrogen and carbon atoms
are broken and replaced with bonds to oxygen atoms. Notice that the bond
energy of oxygen to hydrogen (in water) is higher than the bond energy
of hydrogen to carbon (in propane) 111 kcal vs 98.7 kcal. Especially notice
the double bond energy of carbon to oxygen is more than twice as strong
as the bonds between carbon atoms (192,000 calories per mole vs. 82,900
calories per mole). As bonds are broken, energy is absorbed. As bonds are
formed, energy is released. This shows that the bonds forming as oxygen
connects with hydrogen and carbon atoms produces more energy than what
was needed to break the bonds. Therefore, we've got fire (heat & light).
The propane heater in this hot air balloon depends on the bond energies
of C=O and O—H being higher than C—H and C—C. It's that difference which produces all the heat needed. Below is a detailed account.
Propane Combustion Reaction
C3H8 + 5O2 --> 3CO2 + 4H2O
You can also look up the heat of combustion of propane. It has the same answer, but it was obtained by actually measuring the heat generated from burning propane. We did it without burning propane, but by just adding up the bond energies needed to break bonds and the energy that would be released as bonds are formed.
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
P |
|
Energy absorbed to break bonds |
Energy Released by bond formation |
|||||||||||||||
| 1 | Propane bond energies (kcal/mol) |
CO2+H2O bond energies |
||||||||||||||
| 2 | 98.7 | 191 |
191 |
|||||||||||||
| 3 | 98.7 | x |
2 |
98.7 | O |
= |
C |
= |
O | |||||||
| 4 | x |
3 |
H |
x |
3 |
O |
= |
C |
= |
O | ||||||
| 5 | H | \ |
82.9 |
| |
82.9 | / |
H | O |
= |
C |
= |
O | ||||
| 6 | H | — |
C |
— |
C |
— |
C |
— |
H | 110 |
110 |
xxxxxxxxxx | ||||
| 7 | H | / |
| |
\ |
H | H |
— |
O |
— |
H | ||||||
| 8 | H |
H |
— |
O |
— |
H | ||||||||||
| 9 | O2 bond | 116 | x5 | H |
— |
O |
— |
H | ||||||||
| 10 | O |
= |
O |
H |
— |
O |
— |
H | ||||||||
| 11 | 8 (H-C) bonds @ 98.7 =790 2 (C-C) bonds @ 82.9 =166 5 (O=O) bonds @ 116=580 Total = 1536 |
6 (C=O) bonds @ 191=1146 8 (H-O) bonds @110=880 Total = 2,026 |
||||||||||||||
| 12 | 1536-2026= -490 kcal energy released per mole propane |
|||||||||||||||

Copper metal from copper ore
2Cu2O(s) + C(s) --> 2Cu(s) + CO2(g)
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
|
| 1 | Copper (I) oxide |
Carbon dioxide |
|||||||||
| 2 | kcal/ mol |
kcal/ mol |
kcal/ mol |
kcal/ mol |
|||||||
| 3 | 70 |
70 |
190 |
190 |
|||||||
| 4 | Cu |
— |
O |
— |
Cu | O |
= |
C |
= |
O | |
| 5 | Cu |
— |
O |
— |
Cu | ||||||
| 6 | Energy to break 4 bonds |
280 |
kcal | Energy released from 2 bonds |
380 |
kcal | |||||
| 7 | 280-380= -100 kcal (energy released) |
||||||||||
CH4 + 2O2 => CO2 + 2H2O

Problem 1: What are the total bond energies that have to be broken in order for methane to burn? (See spreadsheet below)
Problem 2: What are the total bond energies gained from the bonds that form in CO2 and H2O?
Problem 3: What is the energy difference between the bonds broken and those formed in the burning of methane?
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
|
Energy absorbed to break bonds |
Energy Released by bond formation |
||||||||||
| 1 | methane&O2 bond energies (kcal/mol) |
CO2+H2O bond energies (kcal/mol) |
|||||||||
| 2 | H |
191 |
191 |
||||||||
| 3 | 98.7 |
| |
98.7 | O |
= |
C |
= |
O | |||
| 4 | H |
— |
C |
— |
H |
||||||
| 5 | 98.7 | | |
98.7 | 110 |
110 |
||||||
| 6 | H |
H |
— |
O |
— |
H | |||||
| 7 | 116 | H |
— |
O |
— |
H | |||||
| 8 | O |
= |
O |
||||||||
| 9 | O |
= |
O |
||||||||
| 10 | Total bond energies
|
Total bond energiesl
|
|||||||||
| 11 | Difference |
||||||||||

Problem 5a: Again, referring to nitrogen, for my Costello hole-punch structure, how many holes would be filled with electrons?
Problem 5b: How many vacancies (holes) would be showing?
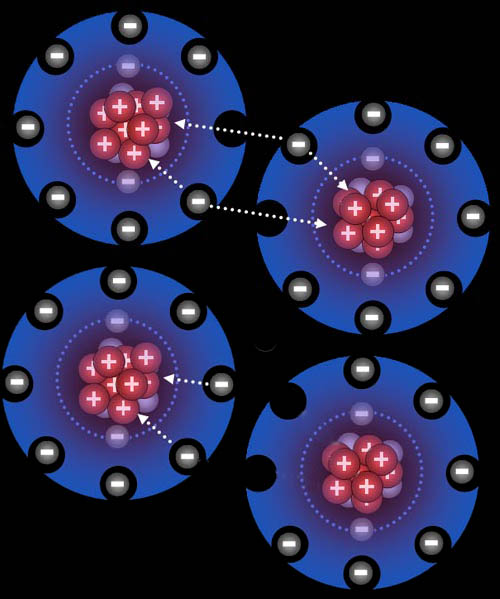
Problem 6: In this fluorine atoms diagram, the electrons from each fluorine atom are being pulled on by the positive nucleus from the neighboring fluorine atom. What keeps one fluorine atom from pulling off one electron from the other fluorine atom (bottom image)?

n=1 |
n=2 |
n=3 |
|||||||||||||||||||
l=0 |
l=0 |
l=1 |
l=0 |
l=1 |
|||||||||||||||||
1s2 |
2s2 |
2p6 |
3s2 |
3p6 |
|||||||||||||||||
|
|
|
|
|
|
||||||||||||||||
m=0 +½,-½ |
m=0 +½,-½ |
m=-1 +½,-½ x |
m=0 +½,-½ y |
m=+1 +½,-½ z |
m=0 +½,-½ |
m=-1 +½,-½ x |
m=0 +½,-½ y |
m=+1 +½,-½ z |
|||||||||||||
Below is Electron Configuration for Cl before gaining electron
n=1 |
n=2 |
n=3 |
|||||||||||||||||||
l=0 |
l=0 |
l=1 |
l=0 |
l=1 |
|||||||||||||||||
1s2 |
2s2 |
2p6 |
3s2 |
3p5 |
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
m=0 +½,-½ |
m=0 +½,-½ |
m=-1 +½,-½ x |
m=0 +½,-½ y |
m=+1 +½,-½ z |
m=0 +½,-½ |
m=-1 +½,-½ x |
m=0 +½,-½ y |
m=+1 +½,-½ z |
|||||||||||||
Problem 8: Sodium is not attracted to chlorine, but after chlorine takes sodium's outer electron, they both become strongly attracted to each other. Why is that?