
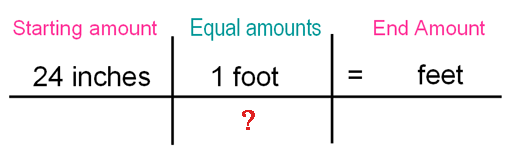
Problem 1:
What goes where the "?" in order to complete this conversion.
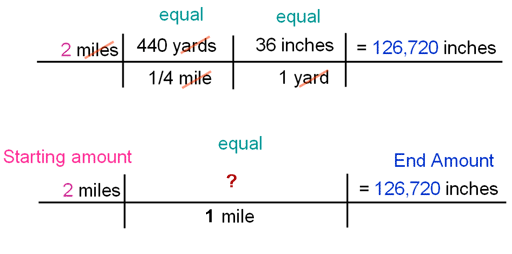
How many inches in 2 miles?
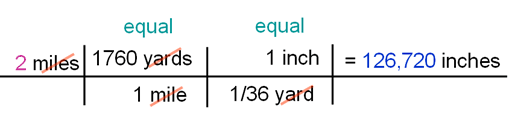
In the tutorial, I converted miles to inches by going through two steps. I knew 440 yards was equal to 1/4 miles and I knew 36 inches equaled a yard. You can also do this conversion in one step. You just have to look up how many inches in a mile. In Google or other search engine, search for "inches in a mile". Problem 2: So what would you write in place of the "?" to do the conversion?
1 ÷ 1 = 1 x 36
36 1

Mass to volume: Let's say you want to know the volume of the iron in a car that gets crushed and melted down. There's 3,000. lbs of iron in a car and iron's density is 7.874 g/cc. You calculate the volume as 173,200 cc using iron's density (see spreadsheet layout below).
Problem 4a: What goes in F2 and G2?
Problem 4b: What goes in F3 and G3?
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
|
1 |
mass of car |
Conversion |
Conversion |
Iron's density is 7.874g/cc |
||||||
2 |
3000. |
lb | 1 |
kg | 1000 |
? |
? |
= |
173,200 |
cc |
3 |
2.205 |
lb | kilo |
? |
? |
|||||
4 |
||||||||||

Note the dimensional analysis below. It begins with the concentration, which is mass per volume. That it multiplied by the volume of the vat. Normally, that is all that is needed to find mass because the volume cancels. However, that only works if volumes have the same units and the mass is the unit you want. Unfortunately, our units for volume don't match (mL vs. gallons). Also grams is not the weight we want. We want the weight in the number of 25 pound bags. See below dimensional analsysis that shows how to solve this.
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
|
1 |
malt concentration mass/vol |
Volume of vat |
Conversion |
Conversion |
Conversion |
||||||||
2 |
2.5 |
g | 1000. |
gallons | 3785 |
mL |
1 |
lb |
1 |
bag | = | ??? |
bags |
3 |
100 |
mL | 1 |
gal |
454 |
g |
25 |
lb | |||||
4 |
|||||||||||||
Problem 5b) What units remain?
Problem 5c) How many 25-lb bags are needed (round to nearest bag) (L2)?
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
|
1 |
honey concentration mass/vol |
Volume of vat |
lb to grams
|
Use density to get grams to mL |
Conversion |
|||||||
2 |
3.0 |
lb | 1000. |
gallons | ? |
? |
? |
? |
0.001 |
= | ??? | liters of honey |
3 |
9.0 |
gallons | 1 |
? |
? |
? |
milli |
|||||
lb to grams |
Problem 6a) What goes in E2? | 6b) What goes in F2? | 6c) What goes in F3? | |
density |
Problem 7a) What goes in G2? | 7b) What goes in H2? | 7c) What goes in G3? | 7d) What goes in H3? |
Answer |
Problem 8) How many liters of honey is needed | |||
| Formula |
Problem 9) What is the spreadsheet formula that goes into K2? (hint: It starts like this: =A2*C2*..." | |||