When you think of fasteners, you probably think of nuts and bolts. Surprisingly, we haven't done much improvement on fasteners for a couple of thousands of years.
The problem with our fasteners are that they aren't flexible. In other words, you can't use a 1/4 inch nut on a 1/2 inch bolt. You can't even use a 1/4 inch nut on a 1/4 inch bolt if the threads don't match (There are about 3 or more types of threads).

Another type of connector are tubing connectors. I like to use PVC tubing and connectors to make stands and other objects. Unfortunately, these kind of connectors are very limited. So that limits what you can build with them. You also have the problem of different diameters. 1/2 inch, 3/4 inch, 1.5 inch, etc.
In general all of the fasteners and connectors that engineers have built so far "stink".
We need to learn from electrons. They are the ultimate masters in knowing how to connect things together.
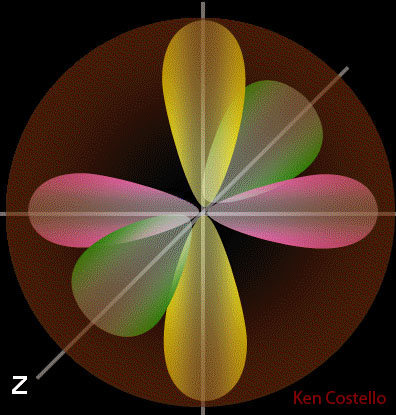
For example, here are five d orbital electrons. Notice how they pass through each other. They can also connect at various angles.
No fastener or connector that we've ever built can pass through each other.
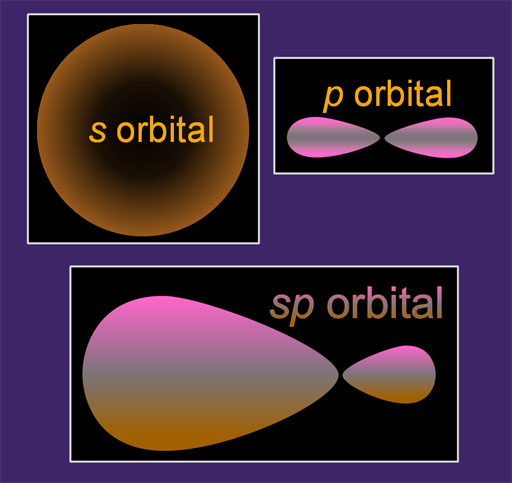
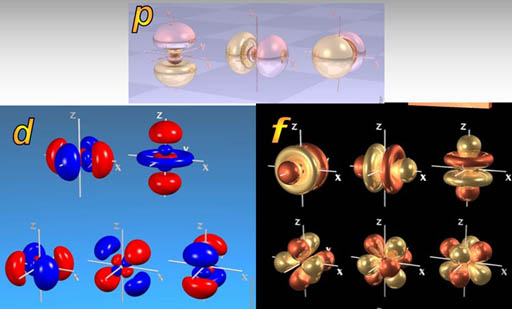
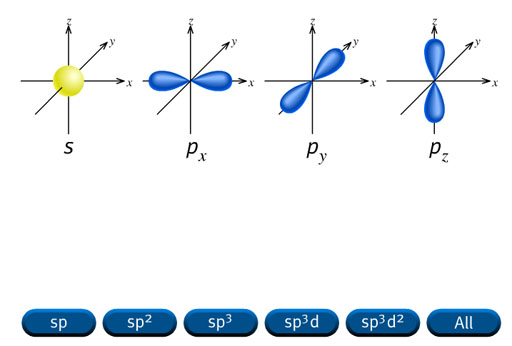
Up until now we have talked about the s, p, d, and f orbitals. That alone gives atoms all kinds of flexibility in connecting with other atoms. This tutorial is about how these orbitals can blend with each other to form new shapes, which gives even more flexibility in bonding. This blending is called hybridization. It's a good word because the final orbital is a hybrid of the original orbitals.
In my example on the left, I show an s orbital and a p orbital at the top. When they blend they make an sp orbital. You can see some of the traits of both s and p orbitals in the sp orbital.

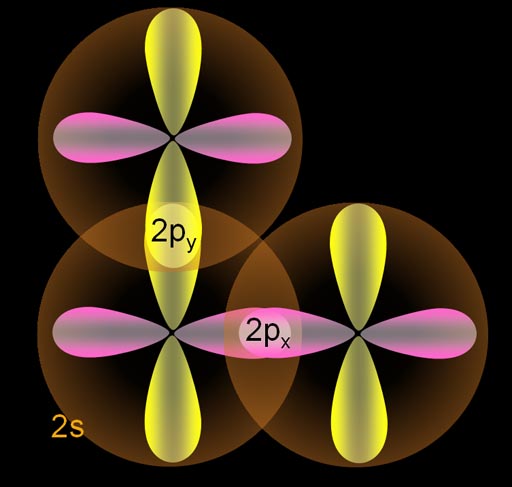
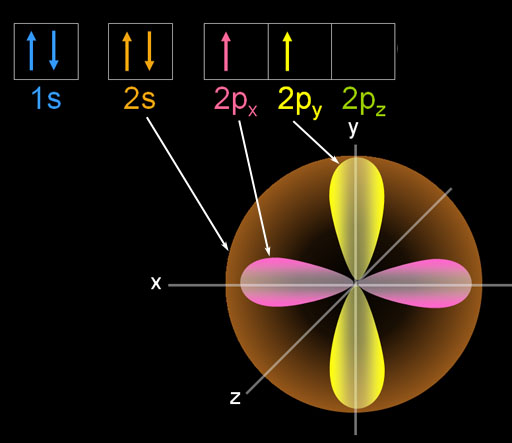
Here is the ground state of carbon. The 1s electrons are small and not shown. They aren't involved in bonding. The two paired 2s electrons are spherical and are symbolized with two arrows. [↑↓]. There are 2 unpaired p orbital electrons that align on the x and y axes.
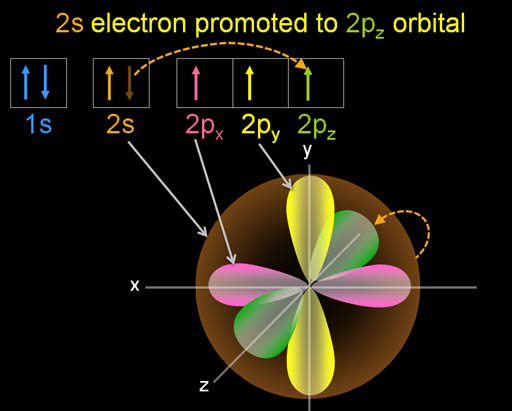
As the carbon atom nears another atom, such as hydrogen, one of the electrons in the 2s orbital gets pulled into a higher energy orbital. In this case, it goes into the unfilled 2pz orbital (see next panel).
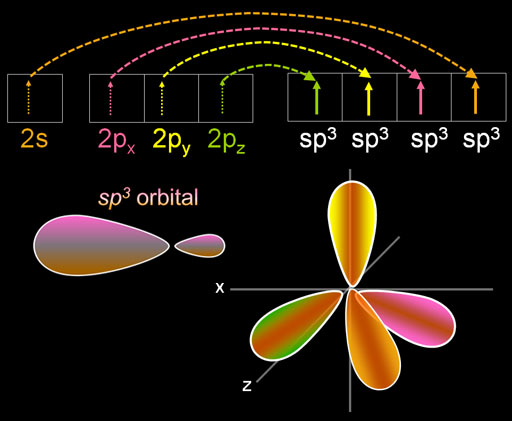
What happens next is that these four electrons blend with each other (hybridize), and they all become the same shape. The shape is two lobes with one much larger than the other. In the right image I'm showing the four larger lobes and not the small ones.
The shape is named after the number of the orbitals involved. The name "sp3" means there was one s orbital and three p orbitals that got hybridized. Warning: In the electron configuration symbols of "2s22p3", the exponents are the number of electrons. In "sp3" the exponent is number of orbitals. There can be 4 to 8 electrons in the "sp3" orbitals. Notice in the image the 4 electrons in the sp3 orbital area unpaired. If all were paired, you would have 8 electrons (the octet).
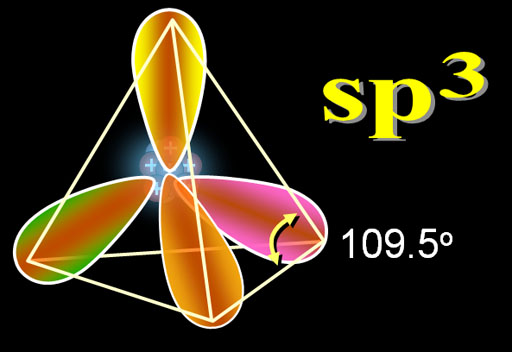
When you see the zigzag lines, those are carbon atoms connecting with the sp3 hybrid electrons. The angles are 109.5°. Even if the lines are drawn straight like the red ones in the image, they are still 109.5°.
The 3 dimensional ball-and-stick model below also shows the tetrahedral angles of 109.5 degrees due to the sp3 electrons.
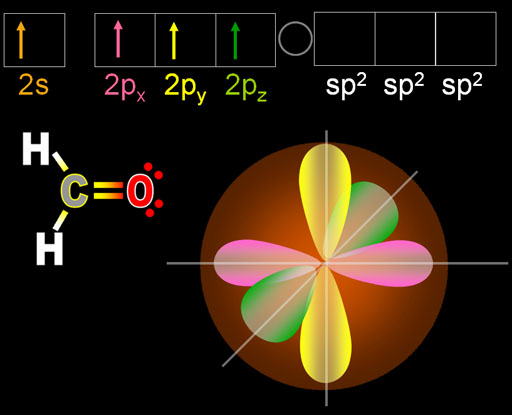
When carbon is surrounded by 2 hydrogen atoms and an oxygen atom, carbon's electrons will do something different. Remember oxygen needs 2 electrons to make an octet, so carbon needs to share two electrons with oxygen by forming a double-bond with oxygen. At the top I show the electron configuration of carbon after one of the 2s orbital electrons have been promoted to the 2pz orbital.
Three electrons are going to get hybridized. From the name of "sp2" you can deduce that it will be one s orbital electron and two p orbital electrons.
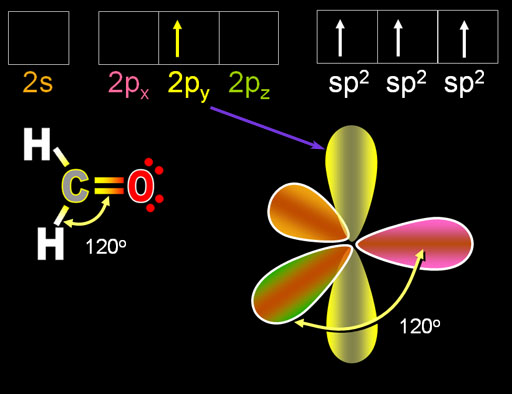
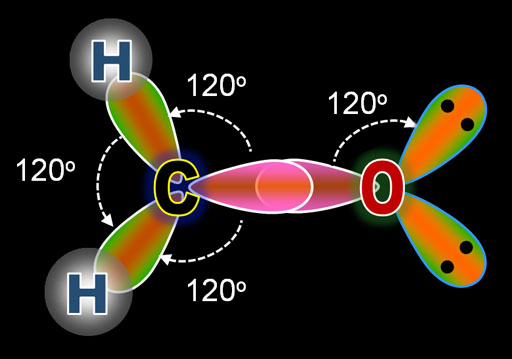
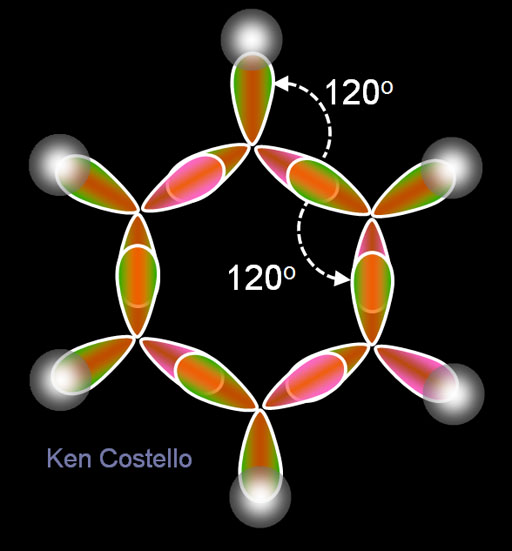
This image shows the result of 3 of carbon's electrons hybridizing into sp2 electrons. Notice these three sp2 electrons spread out in a triangle with 120° between them. In the earlier tutorial on VSEPR (Valence Shell Election Pair Repulsion) we saw that when a central atom bonds with 3 other atoms and there's no lone pair on the central atom, the shape of the molecule is called planar triangular. This hybridizing is how that happens.
The one 2py electron (yellow lobes) did not get hybridized which is how carbon forms a double bond with oxygen shown next.
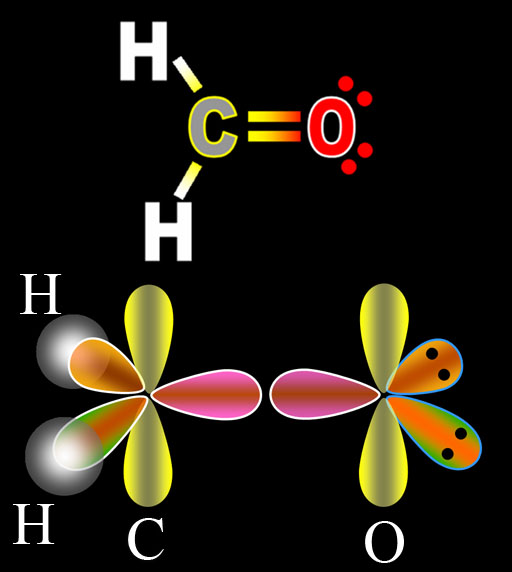
The image on the left is of carbon combining with 2 hydrogen atoms and one oxygen atom to make formaldehyde. The top diagram is the Lewis Dot Structure. The bottom illustration shows the orbitals as they prepare for bonding. Notice that the oxygens electrons underwent the same hybridization as did carbon's electrons. The presence of carbon triggered oxygen to also do hybridization. The difference is that oxygen has 2 more electrons than carbon, which explains why two of the sp2 orbitals are full. That's indicated by the 2 black dots, which represent the 2 paired electrons. Since these are not used for bonding, these are called lone pairs.
Now we will see how the bonded molecule looks...
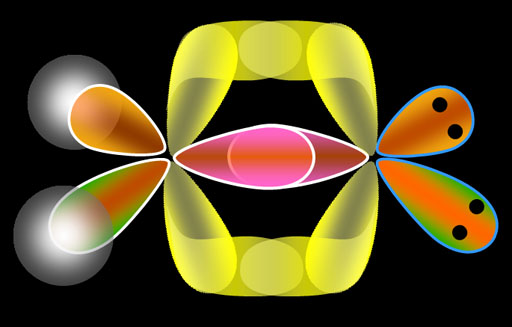
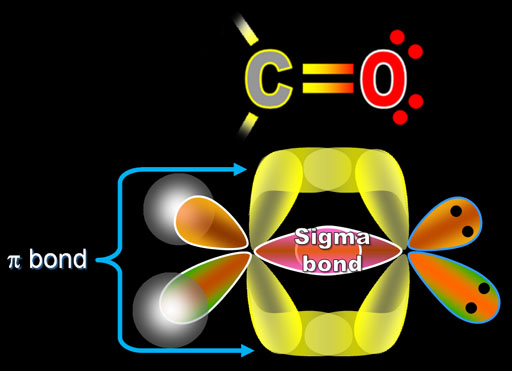
By the way, bonds that overlap directly between 2 atoms are called sigma bonds. The overlapped region is spherically shaped, much like the s orbital. The Greek letter for the "s" sound is σ (sigma). The sigma bonds are the strongest of the bonds.
The bond formed by the overlapping p orbital electrons is called a pi (π) bond. Note the overlapping at the top and bottom of the p orbital electrons, but it still counts as just one pi bond. In the Lewis Structure at the top, the double bond symbol (=) shows 2 lines. One is the sigma bond and the other line represents the pi bond.
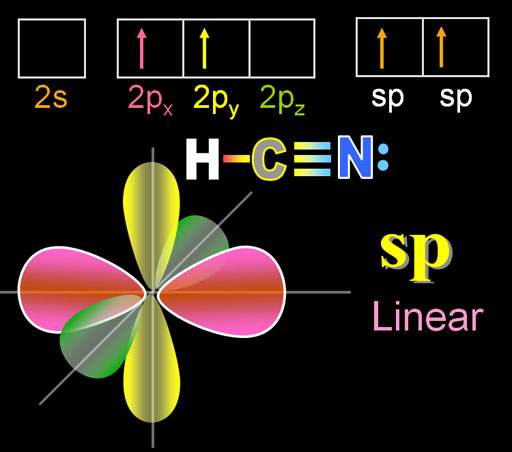
To show its flexibility even more, carbon can connect to an element that needs 3 electrons to reach its octet. The molecule of hydrogen cyanide has carbon in the center of hydrogen and nitrogen. A triple bond is needed with nitrogen.
Like before, carbon promotes one of its 2s electrons to the 2pz orbital. Then the other 2s electron plus one of the p orbital electrons gets hybridized into an sp orbital. The two sp orbital electrons point in the opposite direction. One will bond with hydrogen and the other with nitrogen. The two 2px and 2py electrons will form the other two bonds. (Shown in next panel.)
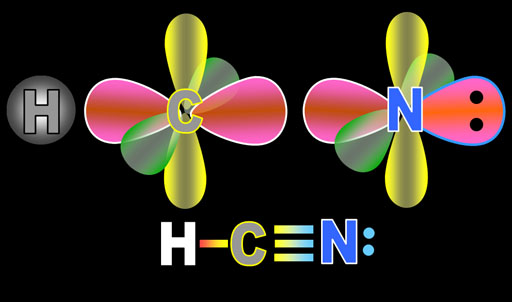
Here the hydrogen, carbon, and nitrogen atoms are set to bond. The pink sp electrons point directly at the atom they will bond with. Notice that the electrons in nitrogen did the same hybridization as carbon. The only difference is that nitrogen has 1 more electron than carbon which explains why one of nitrogen's sp electrons is full (2 dots) and forms a lone pair (non-bonding pair).
The yellow lobes are the unchanged p orbital electrons as are the green lobes. They are going to bend towards each other and overlap.
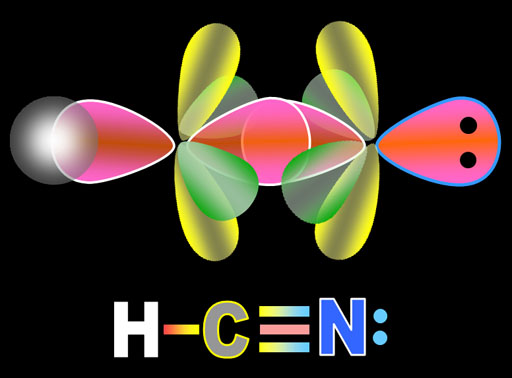
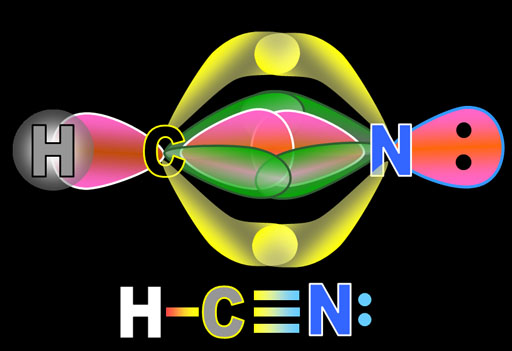
The yellow lobes form a single pi bond above and below the sigma bond. The green lobes form a single pi bond to the front and back of the sigma bond. So in the Lewis structure that shows the triple bond, one line represent the sigma bond and the other 2 lines represent the 2 pi bonds. The lone pair of electrons on the nitrogen atom are the 2 dots in the non-bonding sp orbital. The hydrogen atom also forms a sigma bond with carbon's second sp orbital electron.
The shape of the molecule is linear because the sp electrons are on opposite sides of carbon and nitrogen and there are no lone pairs on carbon to repel these bonds.
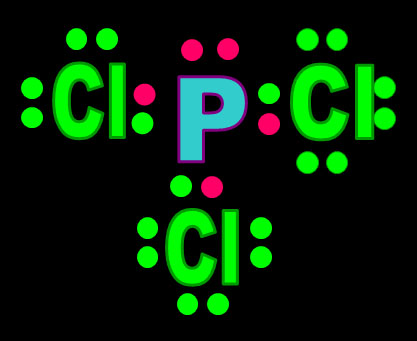
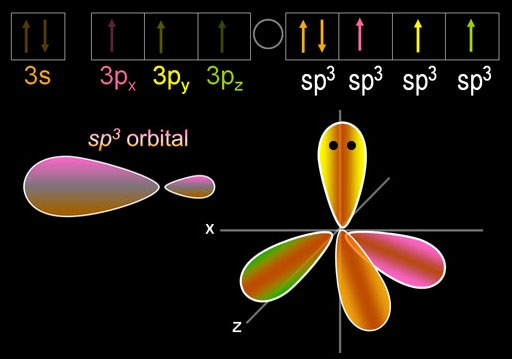
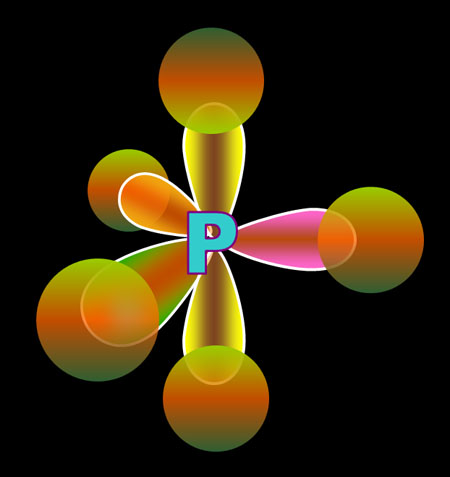
The five electrons are blended (hybridized) to make four sp3 orbitals. Because phosphorus has one more electron than carbon, one of those sp3 orbitals is filled as indicated by 2 dots on the orbital and by [↑↓] in the electron configuration. The other three sp3 orbitals have just one electron in them, so they are available to accept electrons from chlorine and donate one. In other words, these orbitals are ready for bonding.
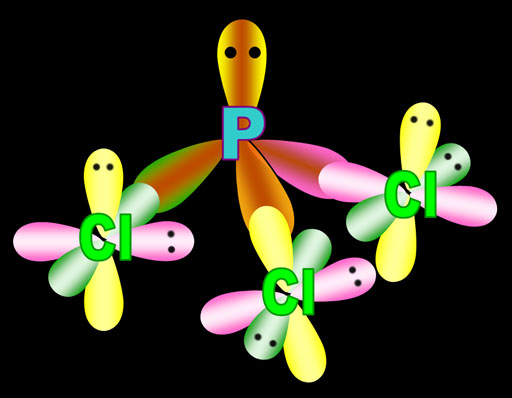
Here I'm showing phosphorus with its sp3 orbitals, that had just one electron, sharing that electron with a p orbital electron from 3 chlorine atoms. The fourth sp3 orbital is full (a lone pair) so it can't bond, but it does repel the other bonds causing the shape of the molecule to be a trigonal pyramid.
![]()
Here I show the chlorine bonding using its only p orbital that isn't full (others have 2 dots). The textbook says the chlorine hybridizes all 7 of its outer electrons also making sp3 electrons. The literature shows it both ways. So we really don't know if chlorine leaves its electrons alone for bonding or hybridizes them. Either way, it doesn't change the angle.
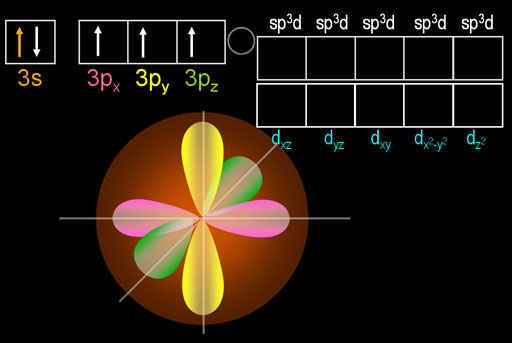
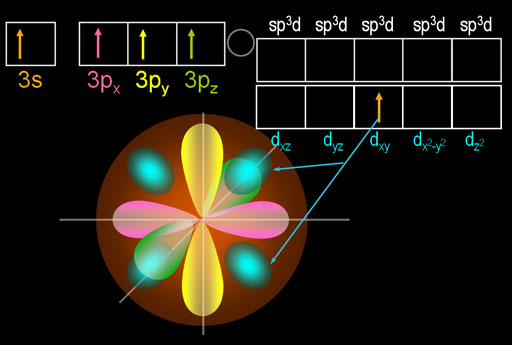
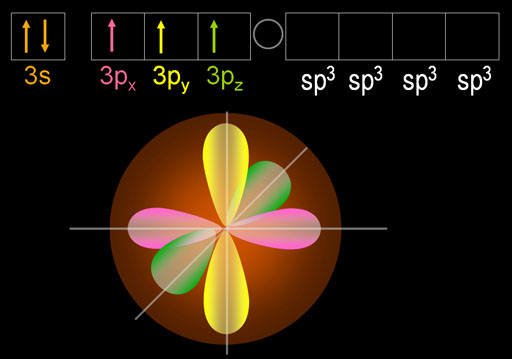
What you see here is that 1 of the 3s electrons got promoted to one of the d orbitals. That causes the appearance of four turquoise egg shape lobes. Those 4 lobes are just one d orbital electron that came from the 3s orbital. I picked the dxy orbital because it's easier to draw.
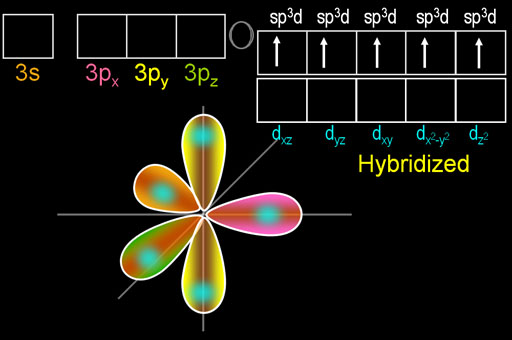
The beauty of this move is that phosphorus now has five electrons that are in 3 different orbitals. That allows it to hybridize all of them into 5 identically shaped orbitals. These are called sp3d orbitals because the one s orbital, three p orbitals, and one d orbital were hybridized.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/hybrv18.swf