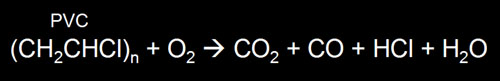| As usual, copy the questions below (highlight
the text, then press the CTRL key plus "c", or use the Edit menu
at top and choose "Copy")
In your email program start composing an email and paste
the questions to your email (use CTRL-V to paste or use Edit menu and
choose "Paste"). You can then answer them in your email and
send it to chm107@gmail.com.
|
| Nylon is a versatile
polymer. There's even a magazine named after it. I assume it's because of
all the clothes and accessories made with nylon. It's only right that nylon
was named after the two cities of fashion where it was invented, New
York and London. |

|
| #1: Below is an animation that
shows two compounds coming together to form a chain of nylon. As they come
together a molecule is given off. What is that molecule? |
|
|
|
#2: In the
tutorial, we talked a lot about ethylene. The Material Data Safety Sheet
(MSDS) for ethylene states that ethylene can undergo "Hazardous
Polymerization." We know it forms a polymer, but how can
it be hazardous? This Web page has a glossary.
www.encyclo.co.uk/define/polymerization
Go down to definition of #6 for polymerization. You will find the meaning for "Hazardous
Polymerization." What is the meaning?
|
|
#3: In the tutorial I didn't cover what can start
the chain reaction of ethylene turning into polyethylene. On the web
page below, the second section "What is
vigorous polymerization," explains how polymerization
can happen.
http://www.ccohs.ca/oshanswers/chemicals/reactive/react.html
Report on how the polymerization might start.
|
| The polymer, polyester, got popular in
the 70's when people wanted to dress cool but didn't want to spend time
ironing and fussing with their clothes. Clothes made from polyester fibers
were just the thing. |
|
| There was even a movie called
"Polyester," because polyester fabrics were "drip-dry"
also called wash and wear meaning they were quick to wash, quick to dry,
and no ironing needed. This freed you to have fun rather than doing household
chores. |
|
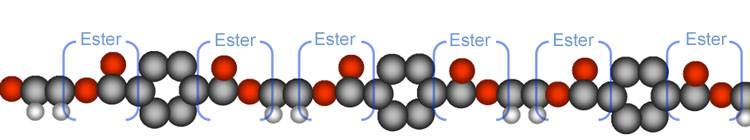
| When you see
the word, "polyester," you should think "Many" "Esters."
Below you see the parts of the polyester polymer that gives it its name.
Esters are compounds that have an carbon bonded to two oxygens and one of
those oxygens is connected to another carbon. |
|
|
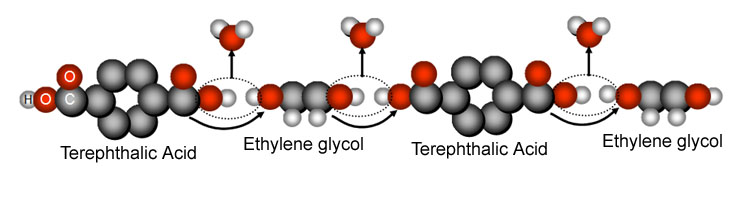
Below we see
how polyester is made. It is made by a chain reaction of two compounds.
Question #4: What molecule is released as these two molecules connect? |
|
|
| Question
#5: One of the two compounds to make polyester is "ethylene glycol."
It a common compound- poisonous, too. But you find it in automobiles. Do
a search and tell me what is the common name for this compound? (hint: add
"automotive" to the search) |
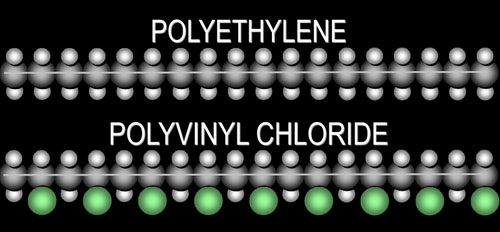
| Another polymer, which is almost the same
as polyethylene, is PolyVinyl Chloride or PVC. The
difference is that every other hydrogen is replaced with a chlorine atom.
|
 |
| PVC pipes are used in our homes and they are
even handy for making a table or chair. PVC is also used as insulation around
electric wires in the home and the automobile. PVC is quite safe until it
burns. The chlorines in the PVC combine with the hydrogens in the PVC to
form hydrogen chloride gas (HCl). When this contacts water in lungs or mouth,
it turns to hydrochloric acid. |
|
| Question #6: To the right is a chemical equation
that shows the combustion of PVC. Name the four products (compounds to the right of the arrow) and identify which
two are safe and which two are toxic. |
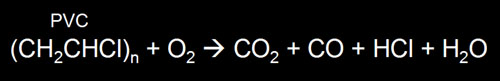 |