
You may remember this collage of things that relate to hydrogen peroxide (H2O2). The only difference between all of these is the concentration of hydrogen peroxide. The jet pack and rockets use 90% w/v. Bleaching of the hair uses between 3% w/v and 6% w/v. The hydrogen peroxide put on cuts is 3% w/v. Labs often keep 30% w/v solutions around.
Problem 1: How many mL of 30% w/v hydrogen peroxide would you need to make 550mL of 3% w/v hydrogen peroxide (H3)?
(Hint 550mL and 3% are the two knowns given)
A |
B |
C |
D |
E |
F |
G |
H |
I |
|
| 1 | Given concentration and volume (weaker) |
Stronger inverted |
|||||||
| 2 | 3% w/v |
||||||||
| 3 | 3 |
g H2O2 | 550 |
mL of weaker | 100 |
mL of stronger | = | ?? | mL |
| 4 | 100 |
mL of weaker | 30 |
g H2O2 | |||||
(This time the given was the stronger solution. Also instead of asking for volume of weaker, it's asking for the concentration. Don't include the 100 at G4 in calculation, that 100 stays and becomes I4)
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
|
| 1 | Given concentration times volume to get grams H2O2 |
weaker mL placed to have vol in denominator |
makes answer per 100mL |
Final concentration after dilution | ||||||
| 2 | 90% w/v |
vol of stronger |
||||||||
| 3 | 90 |
g H2O2 | 10 |
mL of stronger | 100 |
= | ?? |
g | ||
| 4 | 100 |
mL of stronger | 250 |
mL of weaker | 100 |
100 |
mL wealer | |||

A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
|
| 1 | Given concentration times grams of solution to get grams H2O2 |
total grams of solution after dilution put in denominator |
makes answer per 100g |
Final concentration after dilution in %w/w | ||||||
| 2 | 30% w/w |
mass of stronger |
||||||||
| 3 | 30 |
g H2O2 | 330 |
g strong solution | 100 |
= | ?? |
g H2O2 | ||
| 4 | 100 |
g strong solution | 2330 |
g weak solution | 100 |
100 |
g of weak | |||

A |
B |
C |
D |
E |
F |
G |
H |
I |
|
| 1 | moles per liter times mL gives millimoles HCl |
weaker solution conc. inverted to cancel moles |
Final mL of weaker solution | ||||||
| 2 | 6 molar HCl |
volume of 6M HCl |
|||||||
| 3 | 6 |
moles HCl | 170 |
mL of strong solution | ? |
Liter | = |
??? |
mL |
| 4 | 1 |
Liter of strong solution | ? |
mole HCl | |||||
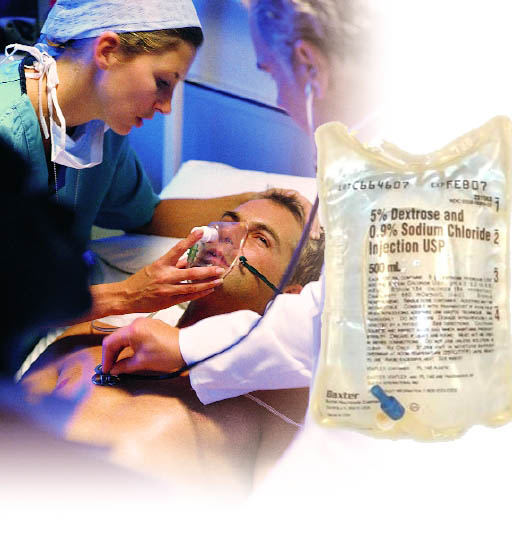
To the left is an IV bag that shows 5% Dextrose and 0.9% sodium chloride. Let's say they got a batch of these IV bags but it didn't have the 0.9% sodium chloride. So the hospital wanted the nurses to use a 20 mL syringe to inject 20 mL of a concentrated sodium chloride solution into the IV bag so that would give the 500mL bag a 0.9% w/v sodium chloride concentration. Problem 6: What is the NaCl concentration needed in the syringe (I3) which will be in % w/v ? (Hint: notice I used 520mL as the diluted volume because of the extra mL added. Also, don't forget to put in the proper number of mL in A4 before calculating.)
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
|
| 1 | Concentration times volume gives grams NaCl |
1mL of strong solution in denominator to end with "per 100 mL" |
makes answer per 100mL |
Final concentration after dilution in %w/w | ||||||
| 2 | 0.9% NaCl |
volume of IV bag |
||||||||
| 3 | 0.9 |
g NaCl | 520 |
mL final solution | 100 |
= | ?? |
g NaCl | ||
| 4 | ??? |
mL weak solution | 20 |
mL of strong solution | 100 |
100 |
mL of strong | |||
Problem 7: What is the formula put in I3?

A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
|
| 1 | moles per liter times liter makes moles |
cancel µ |
Use your atomic # as mL of water, which is final volume |
mole-to-gram with molar mass |
add milli |
Final mg/mL of diluted solution |
|||||||
| 2 | Concentration of strong solution 8.5M |
vol. strong |
|||||||||||
| 3 | ??? |
moles of HgCl2 | 500 |
µL | 10-6 |
??? |
g. HgCl2 | m |
= |
??? |
mg | ||
| 4 | 1 |
Liter of strong solution | µ |
??? |
mL of diluted solution | 1 |
mole HgCl2 | ??? |
1 |
mL | |||
Note at column E, you have got moles of HgCl2. At column G, it is putting those moles over mL of water. So you are basically showing moles per liter of the diluted solution. At column I, this is now grams per mL of solution. At column L, you have milligrams of HgCl2 per milliliter of diluted solution. You have to plug in values for A3, F4, H3, and J4 before doing the final calculation. Remember your answer will be different than anyone else's answer.