
Humans have been familiar with acids and bases for thousands
of years. They didn’t know the exact chemistry, but they knew a lot
about them.
The word acid comes from Latin word
acidus meaning sour,
sharp, or tart taste. Vinegar means sour wine. Vinegar is acetic acid in water plus other substances. Acetum
is an old name for vinegar and comes
from the word acere meaning to be
sour.

Citrus fruits also
have a sour or tart taste due to a different acid
called citric acid.
Sweet-Tarts get their sour or tart
taste from citric acid.

Remember, I mentioned in an earlier tutorial that you can taste the protons from acids. Like this girl's reaction, protons taste tart or sour.
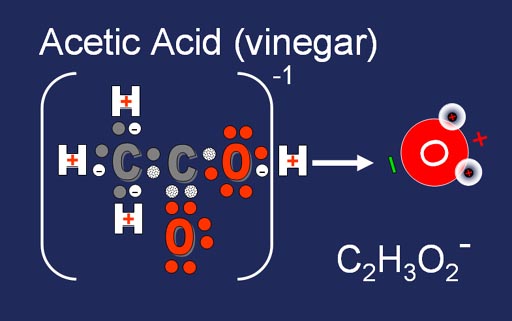
When hydrogen's proton comes off or reacts with something, its electron is left behind. This makes the remaining molecule negatively charged. Acetic acid disassociates into the hydrogen ion (H+) and the acetate ion (C2H3O2-). If water is present, it will grab the H+ ion.

The first definition of an acid is a substance that releases a proton (H+). It's normally released in water, since water is the most common solvent. As an example, hydrochloric acid is shown disassociating into H+ and Cl-. Sometimes the equation is shown with water receiving the H+ making H3O+. In reality, water will absorb the H+, but for simplicity's sake, it's often not shown (top reaction).

HNO3 --> H+ + NO3-
KHC8H4O4 --> H+ + KC8H4O4-
H2C2O4 --> 2H+ + C2O42-
This definition is usually called the Bronsted-Lowry definition of an acid.
In general hydrogen that gets released from any compound (represented by "X") leaves its electron behind, so it comes off as H+ and the "X" part left behind becomes negatively charged X-. An example, would be nitric acid. When the hydrogen comes off, it leaves its electron with the NO3 (nitrate). So the hydrogen is H+ and nitrate is NO3-. The other example is called potassium hydrogen phthalate or KHP for short. That one hydrogen comes off so that makes KHP an acid. Oxalic acid is the bottom one. It loses 2 hydrogen ions (2 protons). More about oxalic acid later in the tutorial.

Power of a Protons' positive (+) charge.
1) A proton attracts electrons
2) A proton is especially attracted to a pair of electrons
2) A proton can push other protons or any atom or group of atoms that are positive.
In other words it has the power to affect other compounds. Sometimes the effect is on their color.
For example, the color of wine or grapes is affected by the acid (protons) present.
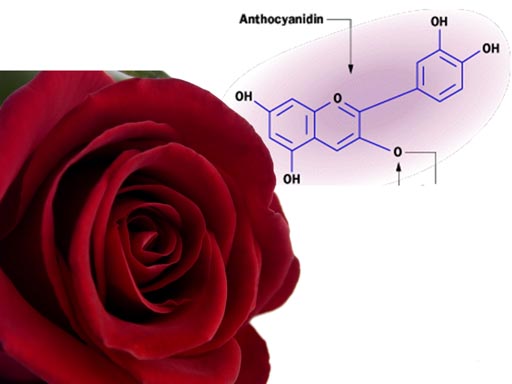
A group of compounds called anthocyanins are responsible for the colors of these plants. It is theorized that this pigment evolved as protection to the high levels of ultraviolet light in Earth’s early history. Besides ultraviolet light, these pigments absorb different frequencies of visible light. This provides plants with different colors.
The color of this pigment is affected by the concentration
of protons (acid level) in the plant. The red of this rose is affected
by the acidity level in the petals. Change the acid levels, and the color
of the rose will change.

Red cabbage has a wide range of color change depending on the pH. In a lab for my CHM107 class, I have students extract the pigment in red cabbage. The pigment belongs to the anthocyanidin family of compounds.


As the pigment is exposed to lower levels of acid and higher alkaline levels of hydroxide (OH-) the color goes from purple to blue to green to yellow to green. Again, the molecule of

The old favorite poem starter "Roses are Red, Violets are Blue..." has a new explanation. The pH in violets is probably different than the pH in roses.

The next time you go by a produce section in the grocery store and see the different colors in the fruits, realize that protons (pH) are partly responsible. In other words, dip them in a strong acid, and they will change color. Dip them in a strong alkaline solution and they will change to a different color.

Compounds are classified as inorganic or organic. Organic compounds contain carbon and are often produced in living things. Inorganic compounds normally have no carbon and are created by chemical reactions without the need for a living organism.
Acids are also classified as organic or inorganic for the same reasons.


Citric acid is produced in citrus (hence the name) and other plants.
The simplest organic acid is formic acid, which is made by ants and makes up their venom.
The way to recognize an organic acid is the presence of a carbon attached to two oxygens with one oxygen attached to a hydrogen. It's called the "carboxylic" group (COOH). The hydrogen has a tendency to be released, which is why it's acidic. Organic acids are not as strong as inorganic acids. For example, only about 1% of the acetic acid molecules lose their hydrogen to water. For the inorganic acids, it’s usually 100%.
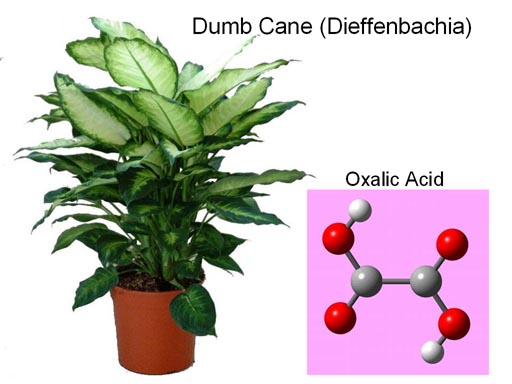
The calcium salt of oxalic acid (calcium oxalate) is found in a house plant called Dumb Cane. The plant was used as a way to punish prisoners and slaves. They were forced to chew one of the leaves. Intense pain in the mouth and throat would follow and the person could not speak (That's why it's called Dumb Cane). Dumb in the sense of deaf and dumb.
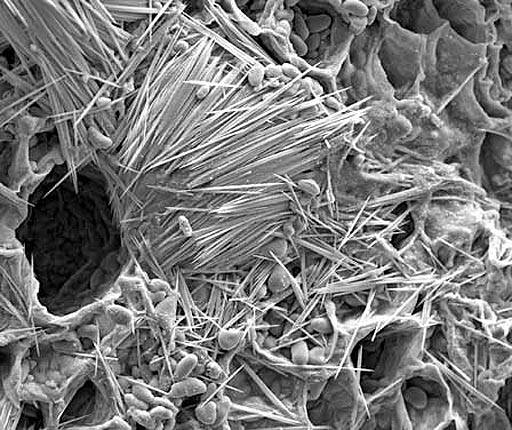
In the leaves of Dumb Cane are cells with needles of calcium oxalate. When chewed, these cells explode and shoot these needles of calcium oxalate into the mouth. So this contributes to the pain from this salt of oxalic acid.
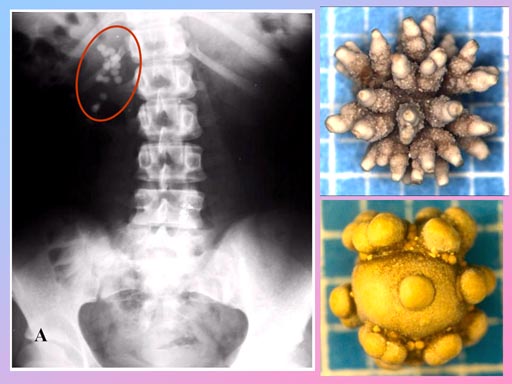
Calcium oxalate is the main ingredient in kidney stones.
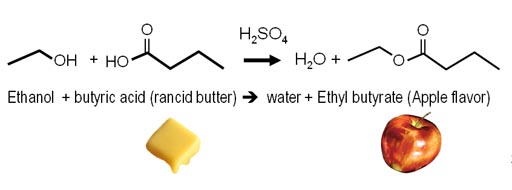
Acids often cause a chemical change.
Remember the synthesis equations I showed on making flavorings? What I didn't mention was that a strong acid, like sulfuric acid, is needed as a catalyst to speed up this reaction. The protons of the acids are attracted to the oxygen atoms, which aids in the release of water and the connecting of the two reactants (ethanol & butyric acid).

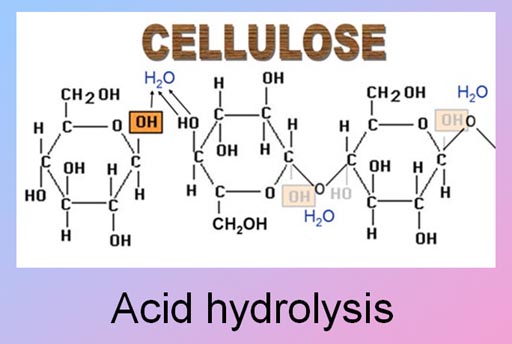
Even though acids are useful in synthesis reactions, acids are also useful for decomposition reactions.
If you have ever mowed lawns, you might have wished that there was something useful for grass clippings. Acids can turn grass clippings into something valuable: sugar.

So far we focused on acids, but what is the opposite of an acid? The opposite would be something that can neutralize or cancel the acid. The name for the opposite of acid is alkaline or basic. Let's see where the word "alkaline" came from.

Al Kali is the Arabic name for the plant we call the saltwort plant. What was discovered a couple thousand years ago was that the ashes from the saltwort plant had the ability to neutralize the power of acids.
The neutralization mostly came from potassium carbonate but some sodium hydroxide and potassium hydroxide would be present. The hydroxide ion (OH-) would react with the hydrogen ion (H+ to form water. So this neutralized the acid. Anything that could neutralize acids like the Al Kali plant did was called alkali or alkaline.

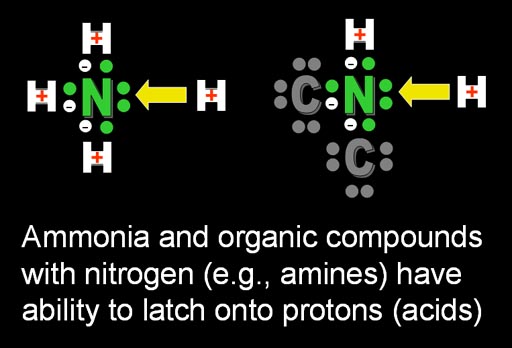
Above the excess H+ ions are taken out of circulation by turning them into HOH (water) by combining with OH-. Another compound that can take H+ ions out of circulation is nitrogen compounds known as amines. That's because nitrogen has an extra pair of electrons that a positive hydrogen will be attracted to. So H+ will latch onto the pair of electrons leaving fewer H+ ions in solution.
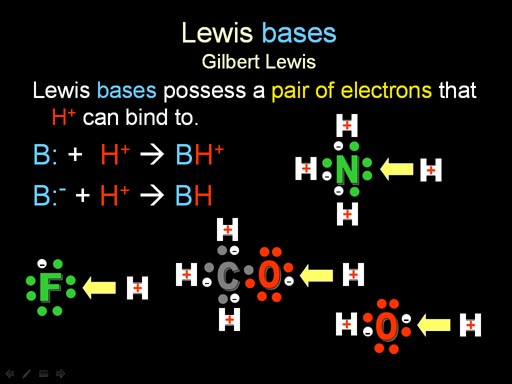
Bronsted & Lowry defined acids as substances that released H+. They also defined bases (alkali) as substances that released OH-.
Lewis Base: Gilbert Lewis broadened the definition of acid and base. A Lewis base possesses a pair of electrons that H+ can bind to, like the amines and alkaloids shown above. Here we see ammonia (NH3), methoxide (CH3O-), fluoride ion (F-), and hydroxide (OH-). They all have a pair of unshared electrons available for H+ to bind to. These unshared pair of electrons are often referred to as a lone pair.
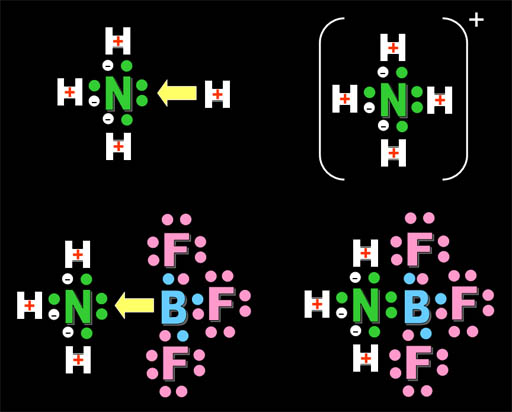
On the top we see the acid (H+) being attracted to a Lewis base (a substance with an unpaired electrons (a lone pair) on nitrogen in ammonia. On the bottom, we see the compound, boron trifluoride, that is also attracted to the same lone pair of electrons. So it behaves like the H+ acid and is called a Lewis Acid. Boron has 3 outer electrons that it shares with 3 fluorines. If boron shares the lone pair electrons of nitrogen, then boron will have a stable octet (eight) outer electrons. That's why boron trifluoride binds with ammonia (bottom right). All atoms have their outer shell full of electrons (hydrogen with two; nitrogen, fluorine, and boron with eight).
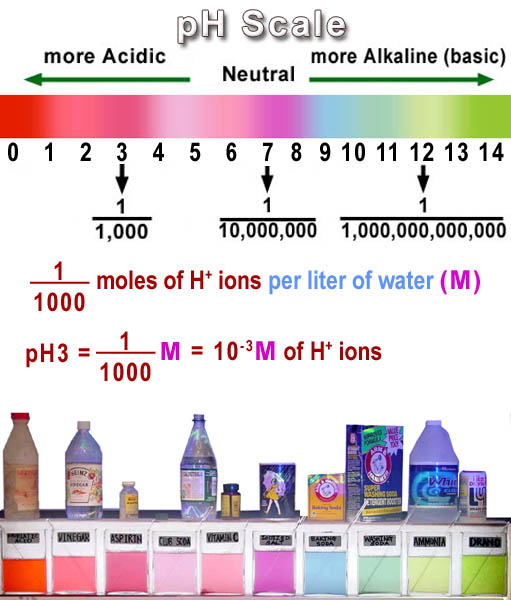
A discussion of acids and bases is not complete without an explanation of "pH". Everyone has heard of pH but few actually know what that means. First of all "pH" gets its name from "potential for Hydrogen" more specifically, the hydrogen ion (H+). So it's a scale that reflects the concentration of H+.
The numbers in the scale is counting the number of zeros in the denominator of a fraction. So a neutral pH of 7 is 1 over 10,000,000 (which has 7 zeros). pH 3 means 1 over 1,000 and so forth. Being a fraction, the more zeros the smaller the number. 1/1,000 is a bigger amount than 1/10,000,000, right? So the scale is reverse. Higher pH means smaller amounts of H+.
So we have the fraction, but a fraction of what? Well, it's the preferred way of counting in chemistry, which is the mole. Since this is concentration, it's moles per liter (molar=M). So pH is counting the moles of the H+ ion per liter but as a fraction. So pH3 is 1/1000 mole of H+ ions per liter. This is also written as 0.001 moles/liter (M) or 10-3 moles/liter (M).

The quick way is to put 1 over 1 followed by five zeros ( 1/100,000). So the concentration is 1/100,000 moles per liter. As a decimal fraction, it's 0.00001 M. The other way is to put the negative of the pH number as the exponent of 10. So pH5 is 10-5M (moles per liter H+).

This healing cream is listed as having pH 5.5. What is the concentration of H+?
Our shortcut doesn't work because we can't write out 5.5
zeros, but we can use the other method of changing it to -5.5
and putting it as the exponent of 10. 10-5.5.
Putting 10-5.5 into
the calculator will come back as 3.16x10-6, so the concentration is 3.16x10-6
M (moles per liter) or 0.00000316M. So pH 5.5 has less H+ ions
than pH 5.
0.00001000 = pH 5
0.00000316 = pH 5.5
Remember the higher the pH the less H+ concentration. Also,
note that a change from pH 4 to pH 3 is 10 times more H+. For
example pH 2 is 1,000 times more H+ than pH 5. (5-2=3 which
is 10x10x10)
Common item |
pH |
Water |
7 |
Grapes |
4 |
Orange juice |
3.5 |
Grapefruit juice |
3.2 |
Apple juice |
3.1 |
Vinegar |
2.9 |
Lemon juice |
2.3 |
Lime (fruit) |
2 |
Stomach juice |
1 |
1Molar (a diluted strong acid) |
0 |
14M HCl (concentrated) |
-1.08 |
18M H2SO4 (concentrated) |
-1.26 |
Fluoroantimonic acid |
-31.3 |
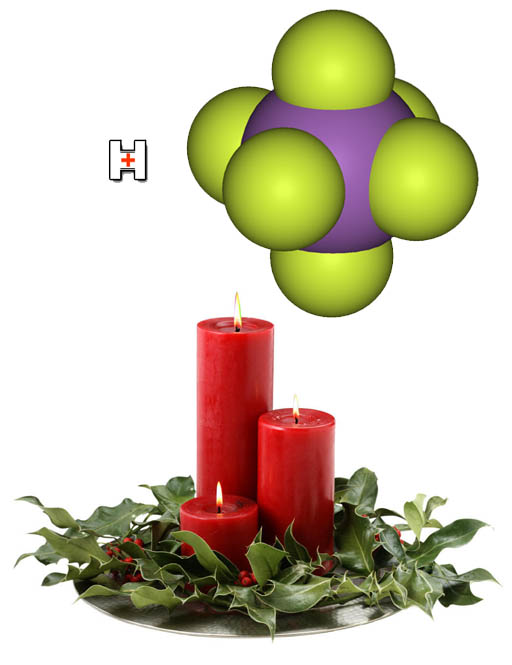
Let's end the tutorial part on a bit of trivia. What is the strongest acid known?
It's fluoroantimonic acid (HSbF6). The yellow
spheres are fluorine and the purple one is antimony (Sb). It's prepared
by adding hydrofluoric acid to antimony pentafluoride.
HF + SbF5 -> HSbF6
This acid is 20,000,000,000,000,000,000 times stronger than 100% sulfuric
acid. It has a syrupy consistency and dissolves pretty much everything.
However, it can be stored in Teflon bottles. In contact with water, it explodes.
If added to hydrocarbons like propane, gasoline, oil, or wax, it causes
them to gain a positive charge making them quite reactive. Imagine a wax
candle that would be dangerous to touch. So even if the acid is gone,
its affect on the wax would make the wax extremely dangerous. In essence
the wax itself becomes a very strong acid, more specifically a Lewis acid
because it will be hunting out a pair of unshared electrons and those
could be in the proteins of your skin.

Problem 1: When you see the word "acid", what ion should you think of?
Problem 2: At what pH do you think the acid concentration is dangerous for the skin?

C6H8O7 --> ? H+ + C6H5O73-
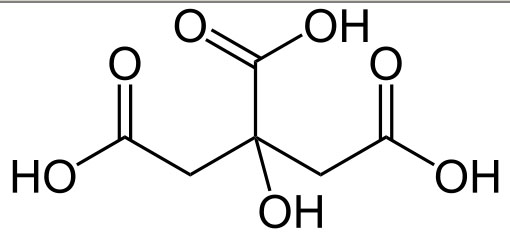
This is chemical equation showing citric acid giving off some hydrogen ions. The structural formula for citric acid is also shown. Problem 4: How many hydrogen atoms came off?
Problem 5: These hydrogens can be pulled off using sodium hydroxide (NaOH). Complete and balance the below chemical equation:
C6H8O7 + 3NaOH --> Na3C6H5O7 + ???
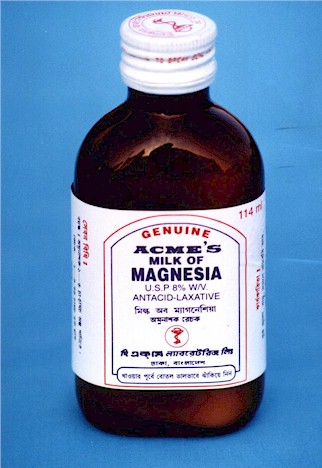
Here is a bottle of Milk of Magnesia, which is magnesium hydroxide. (It's called milk of magnesia because it looks like milk). The formula is Mg(OH)2. The label says it is an antacid and laxative. It's a laxative because the magnesium ions hold onto water in the intestines.
Stomach acid contains hydrochloric acid. In contact with milk of magnesia this reaction occurs that neutralizes the acid: I like to write H2O as HOH to better show the source of hydrogen.
HCl(aq) + Mg(OH)2(aq) --> MgCl2(aq) + HOH(l)
Problem 6: Write the balanced equation.
In water the above compounds are actually ions. So the chemical equation actually looks like this:
H+(aq) + Cl-(aq) + Mg2+(aq) + 2OH-(aq) --> Mg2+(aq) +2Cl-(aq) + HOH(l)
Here we see better how the OH- neutralizes the H+.
Problem 7: The product is magnesium ions, chloride ions, and water. When it dries, it becomes a salt. What is the salt's formula?

Here's a graphic shown earlier. In the middle towards the bottom is a compound known as methoxide (CH3O-). It is quite reactive because of its negative charge. Methoxide is used to attack oils and fats and turn them into biodiesel. In the picture we see that methoxide attracts H+.
Problem 8: What does methoxide turn into after the H+ ion combines with it?
Problem 9: Notice the fluoride ion in the lower left. What does fluoride become after the hydrogen ion combines with it?
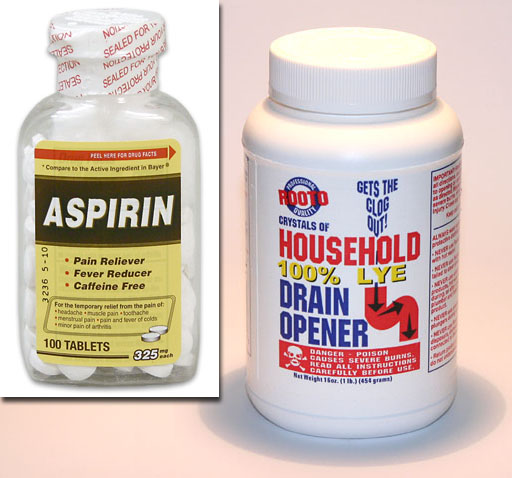
To the left is a bottle of aspirin and a bottle of lye (sodium hydroxide/NaOH). The Lye is a one pound (454 gram) bottle.
Problem 10: How many moles of NaOH is in that bottle?
Problem 11: How many moles of OH- is in that bottle?
On the left is 100 tablets with 325mg each of aspirin. Aspirin is acetylsalicylic acid. It has a molar mass of 180.157 g/mol.
Problem 12: How many moles of aspirin is in that bottle?
Problem 13: Acetylsalicylic acid releases only one H+ ion per molecule. So how many moles of H+ ion is available in that bottle of aspirin?
Problem 14: Of course, one mole of H+ will neutralize one mole of OH-. So how many grams of NaOH is needed to neutralize all the acetylsalicylic acid in the aspirin bottle?
