|
Nomenclature of Common Inorganic Compounds Quiz |
|
Problem 1: Ferrous chloride is used in wastewater treatment. Iron has two oxidation states (3+ and 2+). Ferric refers to iron with a 3+ charge. Ferrous refers to 2+ charge. Those are the old names. This ferrous chloride also has 4 water molecules attached. What is the current name for this compound? |
 |
Problem 2: Here is another old bottle that has the old name for a compound. What is the proper name for "chromium trioxide"?
|
|
Problem 3: This is a bag of potassium nitrate. The company selling it says it is used for making glass, for fertilizing, for gunpowder, for making tobacco paper, and for making medicines. Using Babel Fish, I translated the top 3 characters. The left indicates a nitrate type compound. The middle character means acid. So the first two are saying "nitric acid", but the right character is that for potassium. So this is the potassium salt of nitric acid, which we call potassium nitrate. What is the formula for potassium nitrate and for nitric acid? |
|
White Sands, New Mexico has many miles of sand dunes; however, they are not made from sand. The grains are the mineral gypsum, which is used to make "drywall" for homes. Gypsum is soluble in water, so normally you can't find big "sand" dunes of gypsum, but the low rainfall in the area preserves these grains. The formula for this gypsum is
CaSO4·2H2O
Problem 4: What is the chemical name for this compound? |
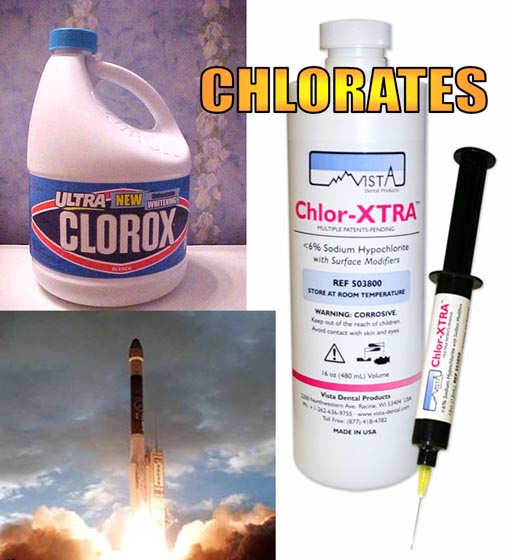 |
These products are using sodium hypochlorite; however, there are other products that replace sodium with a different alkali metal, and those hypochlorite compounds have similar properties to sodium hypochlorite.
Problem 5: What is the name of the hypochlorite compound that uses the alkali metal that is lighter than sodium?
Problem 6: What is the name of the hypochlorite compound that uses an alkali metal that is heavier than sodium but closest to sodium's mass? |

From illuminatingscience.org |
In the tutorial, I mention the heating pad filled with sodium acetate. There's a neat trick that can be done with this supersaturated solution of sodium acetate. If you pour this solution onto a small crystal of sodium acetate, the sodium acetate in the solution that you are pouring will crystallize on top of that crystal so quickly that as you pour the liquid it will turn into a white crystal spire that will look cold but actually be hot. Problem 7: What is the formula for sodium acetate? |
 |
Ammonium hydroxide only exists in water. It comes from ammonia gas (NH3) combining with water (H2O). It is quite toxic because of both the corrosive hydroxide ion and the ammonia gas that can be released.
Problem 8: What is the formula for ammonium hydroxide?
|
 |
Here are 3 bottles of ferric chloride, which is the old name (These bottles are about 30 years old). If you order this compound now, it will show its new name. Problem 9: What is the new name for ferric chloride?
Problem 10: Ferric chloride is used on an industrial-size scale because has many uses. Name one of its uses. |
|
These iron supplements were recalled because the bottle didn't have a child-resistant cap. Iron supplements are dangerous if there's an overdose. The label says it contains "Ferrous fumarate". Fumarate comes from fumaric acid, which is found in mushrooms and moss. Fumaric acid is added to some foods to give them a more tart taste. Most iron supplements use FeSO4. Problem 11: The old name of FeSO4was ferrous sulfate. What is the new name? |
|
|
At one time it was legal to spray salad bar items with a sodium sulfite solution.
This helped preserve the vegetables and fruit and kept them from turning
brown. However, after a few patrons died and others were hospitalized, restaurants
are banned from using sulfites or sulfur dioxide on salad bar items. Problem
12: What
is the health problem with sulfite? |
|
|
Ammonium carbonate is sold here as a leavening agent for pastries. Leavening
causes the dough to rise because it produces gases. Notice all the warnings.
Ammonium carbonate is also used for smelling salts. The formula is
(NH4)2CO3.
Problem 13: What dangerous gas is being released when
this compound is used in baking.
Problem 14: Show the decomposition chemical equation for (NH4)2CO3. |










