A History of the Periodic Table

Chaos, The Ride:
This ride is called Chaos. It's an appropriate name. On the ride, a swirl of images confuses the mind. There's just too many things that you are trying to take in.

Chaos in Life:
There's a movie called chaos. and from their poster is appears to focus on the chaos of life. We can all relate to this. Too many things to grasp and get control of.

Chaos in the Cosmos:
The subtitle says, "The Stunning Complexity of the Universe." Yes, complexity can lead to chaos. Humans are normally uncomfortable with chaos and complexity. They want to simplify it.
One type of complexity is figuring out what everything in the world is made of. For example, are stars, clouds, mountains, rivers, animals, and plants made from something different? Even on an animal, is the horn, teeth, fur, eyes, muscles made from different materials? Is there a way to simplify what millions of things are made of?

Water is the Only Element:
Around 600 B.C., the Greek philosopher, Thales, proposed that all things are made from water. It seemed logical. Water is very abundant. Also, all living things consumed water. So maybe water was being transformed into different shapes and different materials. This belief would really simplify things.

Water and Earth:
Around 500 BC Xenophanes suggested that everything was made from water and earth. Plants needed both water and soil to grow. Animals ate the plants, so they, too, were made from water and earth.
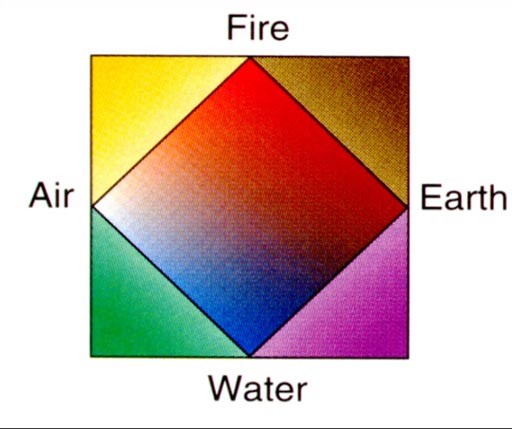
Four Elements:
Around 440 BC Empedocles suggested that there were four elements: Water, Earth, Air, and Fire. It seemed logical because when things caught fire moisture is released, air can be felt coming up from it, and the ashes show the earth that it contained. Classifying all matter as only being made of four elements certainly calmed the chaos of the world.



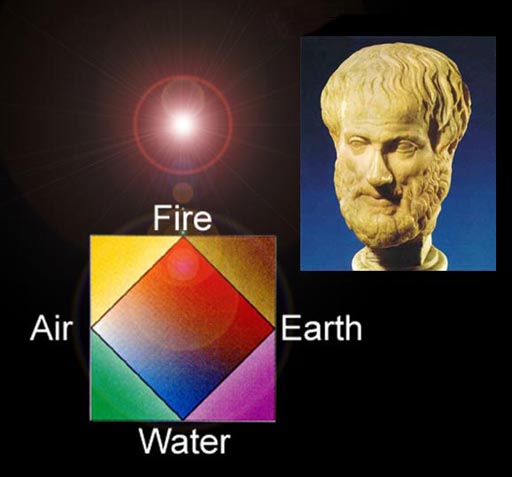
The 5th Element:
Around 340 BC Aristotle said he didn't believe in the theory of Atoms because you are putting a restriction on the gods. If the gods wanted to divide an element to something smaller than an atom, they could.
Aristotle also said the Sun, Moon, & Stars (and all heavenly bodies) were made from a fifth element. This element was so perfect that in contact with base (low value) metals, the fifth element could turn that metal into gold. The fifth element could also cure people and keep them young. This started a 2,000 year hunt for a nonexistent element. So this rather spiritual element didn't really help combat chaos because no one ever found it.

There was even a problem with the other four elements.
The idea of an element is that it is a building block for other things. An element is not supposed to be made from smaller building blocks.
Antoine Lavoisier was a nobleman from France who lived in the 1700's (His wife, Marie, was his assistant). Lavoisier discovered that water is made from hydrogen and oxygen and that air is made from oxygen and nitrogen. So water and air can't be elements because they are built from something simpler. So it was decided that if a substance can be decomposed, then it is not an element.
By the way, Lavoisier is often considered the Father of Modern Chemistry. He wrote the first modern textbook on chemistry.
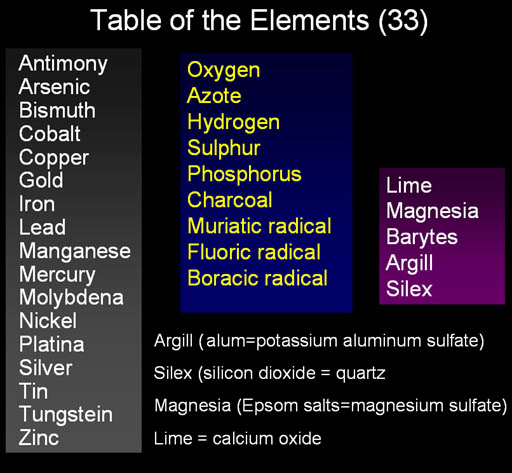
These were the agreed upon elements at the time of Lavoisier. The names in the right purple column were listed as elements but Lavoisier suspected that they weren't elements; he just couldn't get them to decompose. Later, lime was shown to be calcium combined with oxygen. Magnesia consisted of magnesium, sulfur and oxygen. Silex and Argill were also found not to be elements but made up of true elements.

An important point to make here is that science wishes to find the fundamental principles that explain the complexity around us, but it won't stay with a simple view if it finds evidence that requires more explanation. In other words, chemistry didn't stick with these five elements even though it was simple because experiments showed that there were more elements and these were not elements.
Chemistry looks to identify the simple building blocks and fundamental forces, but at the same time it won't ignore evidence that suggests something more complex is going on. So we are always looking for simple explanations but not so simple they can't explain our observations.
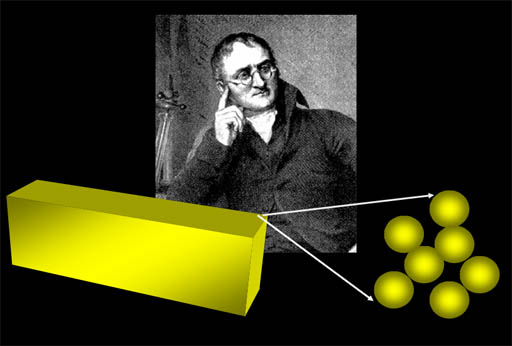

The next idea would tremendously simplify the complexity of all the materials around us. Dalton suggested that substances around us were made up from a grouping of specific number of atoms of different elements. For example, a water molecule is made from two atoms of hydrogen and one atom of oxygen (upper right). If there's 2 oxygen atoms and 2 hydrogen atoms (lower right) then it's not water but something else (hydrogen peroxide). Salt is made from one sodium atom and one chlorine atom. Ammonia is made from 3 hydrogen atoms and one nitrogen atoms (lower left).


At the time of Dalton, these were the elements and the symbols that was used to represent them. The numbers are their relative weights. Azote was the name for nitrogen. It was considered 5 times heavier than hydrogen. That was incorrect because they didn't realize that hydrogen went around in pairs.
Notice the symbols for iron, silver, and gold use the letters I, S, and Gold. That was going to change because of a chemist that was doing a better job at measuring the relative masses of elements.

Johan Jacob Berzelius from Sweden had done 2000 experiments to determine accurate relative masses for the know elements. That apparently gave him the honor of making up the below rules.
Older elements take the symbol from their Latin name.
Fe from Latin word
for iron, Ferrum.
Au from Latin word for gold, Aurum.
Ag for Latin word for silver, Argentum.
Elements discovered in recent times would use symbols based on English name. For example, O for Oxygen.

Researchers had already began to arrange and classify elements in the following ways:
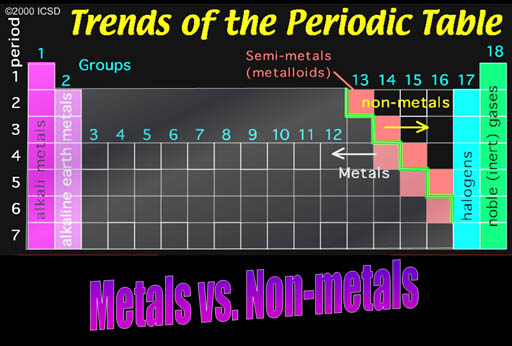
Metals vs. nonmetals
Tables of increasing atomic weight
(like you've seen above)
John Newlands from England had a different way to arrange elements. It was based on his love of music.
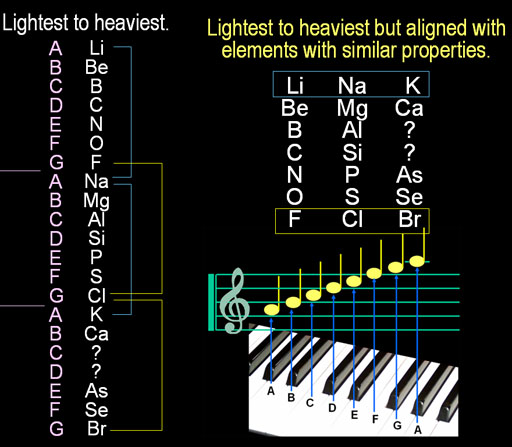
On the left the elements, Lithium, Beryllium, Boron, etc.
are listed from lightest to heaviest. Newlands noticed lithium(Li), sodium
(Na), and potassium(K) all react violently with water and they were 8
apart on the list. Then he noticed fluorine, chlorine, and bromine were
all very corrosive and formed acids. They were also 8 apart on the list.
Coincidence?
It was like octaves in music. Starting with the "A" note, after
8 notes, the "A" note repeats. So instead of a long list, he
arranged them in three columns. Amazingly, the elements in each row had
similar properties with each other. This indicated some hidden connection
between the elements in each row that would simplify our understanding
of this list. Unfortunately, when Newlands presented this to the Royal
Chemistry Society, they laughed at him and told him if he had to rearrange
the elements, try alphabetizing.

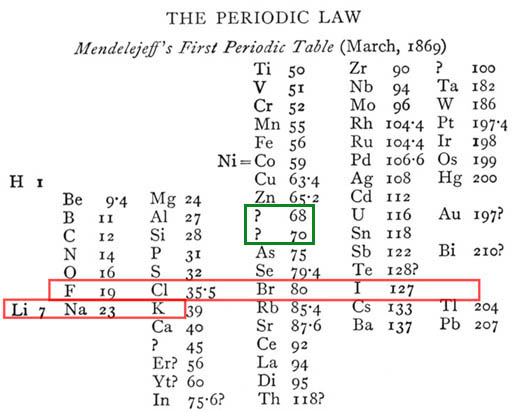

Here is Mendeleev's Periodic Table of the Elements carved in Stone. In this later version, the elements that are related are placed above and below each other like the modern versions of the Periodic Table.
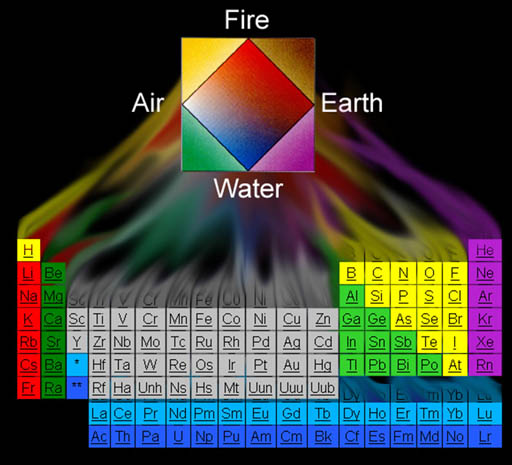
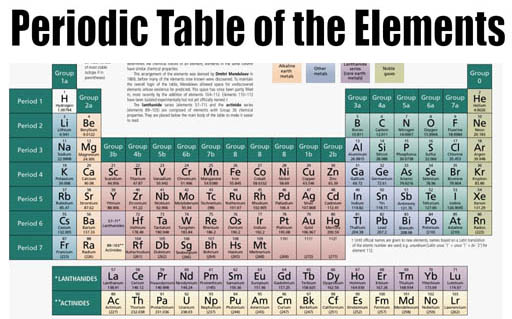
Again, the Periodic Table of the Element has the elements listed by increasing mass but grouped by similar reoccurring (periodic) properties. Some groups are in columns and some are in rows. Let's explain the logic behind these groupings.




Metals also conduct electricity. Notice that the Tesla coil sparks seek out metallic objects because they conduct electricity better than the nonmetallic materials such as wood or soil.
Metals are also malleable and can be bent or hammered into various shapes.
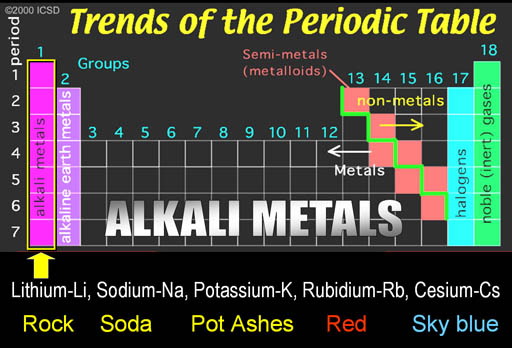
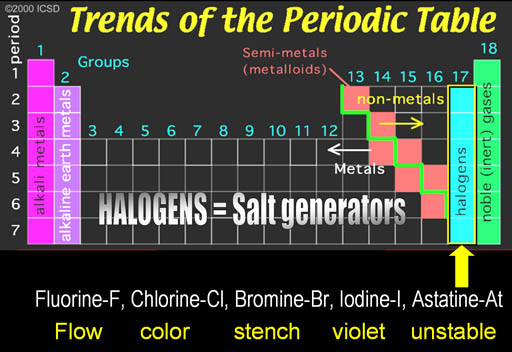
The first group is on the left side of the Periodic Table. The metals in this groups are called the Alkali Metals.
What is usually never mentioned is why these metals are called "Alkali Metals."
Since I don't like to be given names without learning about the origin of that name, I will give you background on these names.
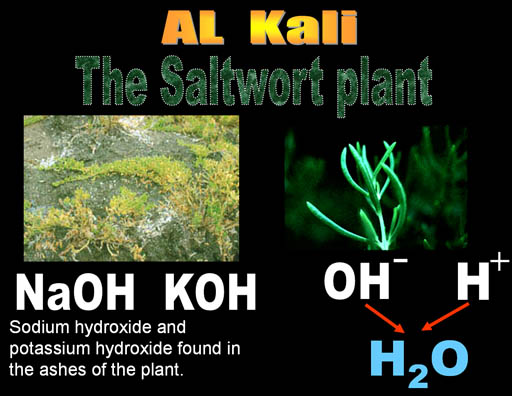
Al Kali is an Arabic word. "Al" means "the" in Arabic. You hear it pretty often. Think of Alcohol, Algebra, and Al Qaeda. The "Al" in all of these mean "the". Kali is Arabic for "Saltwort" plant.
The saltwort plant lives near shores of salty seas. When the saltwort (alkali) plant is burned, the ashes contain sodium hydroxide (NaOH) and potassium hydroxide (KOH). When the ashes are placed in water, the hydroxide (OH-) dissolves in the water and can neutralize acids (H+). Anything that increases the level of hydroxide (OH-) in water is called alkaline. Alkaline batteries contain KOH and it owes its name to the saltwort plant spoken in Arabic (Al Kali).
CaO + H2O --> Ca(OH)2--> Ca2+ + 2OH-

Noble Gases: Helium-He, Neon-Ne, Argon-Ar, Krypton-Kr, Xenon- Xe, Radon-Rn
Noble Gases: The right side group is the noble gases. They were once called the Inert Gases because they were inert and didn't combine with any other elements. However, it was discovered that some could be coaxed into combining some of the very reactive elements such as the halogens.
Along the green stair-step are the metalloids. They have properties similar to metals, such as conductivity. The electronic industry takes advantage of their ability to have their conductivity turned on or off.
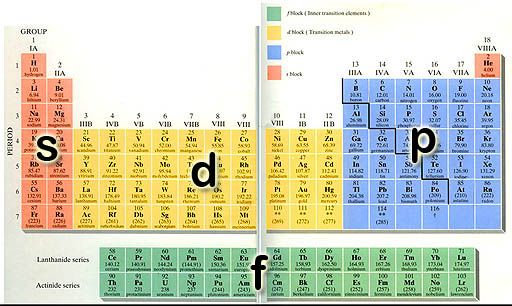
Electron Orbitals: Sometimes a Periodic Table is color-coded to show the four types of electrons that occupy the outer shell (orbit) of the element. An electron orbital is the space that the electrons travel. There's only four of types of orbitals, so this classification of the 100+ elements simplifies the table.
I will briefly go over the 4 types of orbitals below.
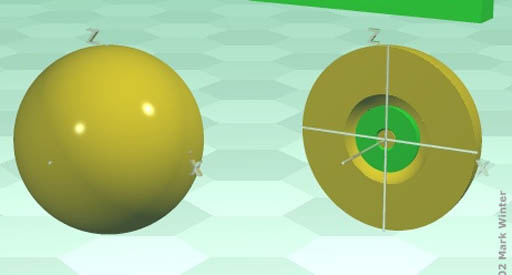
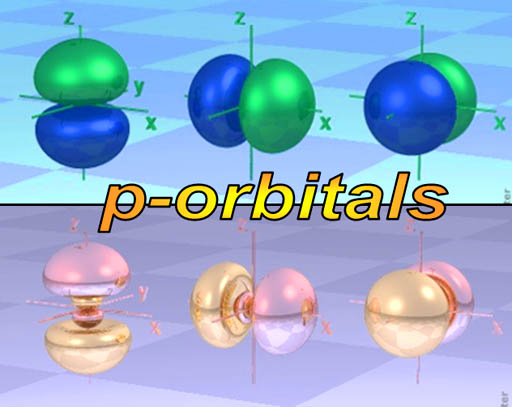
p-orbitals: The elements in the blue p-block above all have their outer electrons in a p-orbital. You might call the shape of p-orbitals dumbbell shape (two lobes). There are basically 3 orientations (x, y, & z axes) of the p-orbital and each can only hold 2 electrons. That's a total of 6 electrons. That's why the p-orbital block is 6 elements wide. (Scroll up to take a look)
The dumbbell shape of the p-orbital makes you think that there's two electrons there. Realize that one electron makes both of these lobes. However, two electrons can share this space, so there might be one or two there.
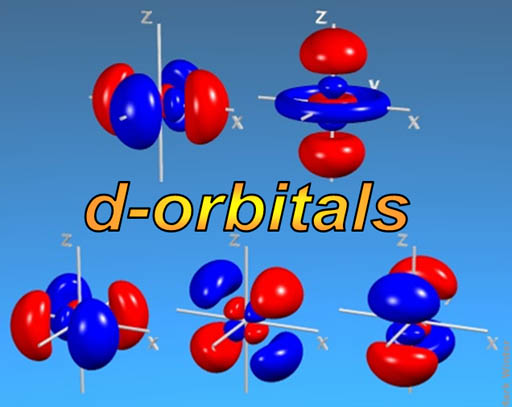
d-orbitals: The middle of the Periodic Table (d-block) is made up of a run of 10 elements. (scroll up to take a look). The reason that there are 10 elements is that there are five orientations of d-orbitals, with each one capable of holding 2 electrons (just like the s and p orbitals). So, again, there's a run of 10 elements as the d-orbitals are getting filled (They are called the transition metals).
I'm not sure how you would describe the shape of d-orbitals. Most are like a double dumbbell (4 lobes). Again, just one electron occupies all of those lobes.
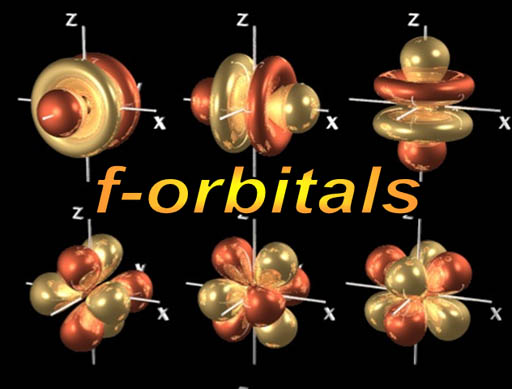
f-orbitals: At the bottom of the Periodic Table are two rows of 14 elements (scroll up and look). There are 14 elements in each row because this is the run of elements as the f-orbitals are getting filled up. As you probably already guessed, there must be 7 f-orbitals with each capable of holding two electrons to explain why 14 elements in the row.
Again, each of these images is one electron. In an element like uranium, there are 92 electrons; 18 of those electrons occupy f-orbitals, 40 are in d-orbitals, 30 are in p-orbitals, 14 are in s-orbitals. All 92 electrons are orbiting together around the nucleus. Somehow they stay of each others way, yet get as close to the protons in the nucleus as possible.
(These beautiful images are by Mark Winter at Sheffield University. Check out his website:
http://winter.group.shef.ac.uk/orbitron/ )


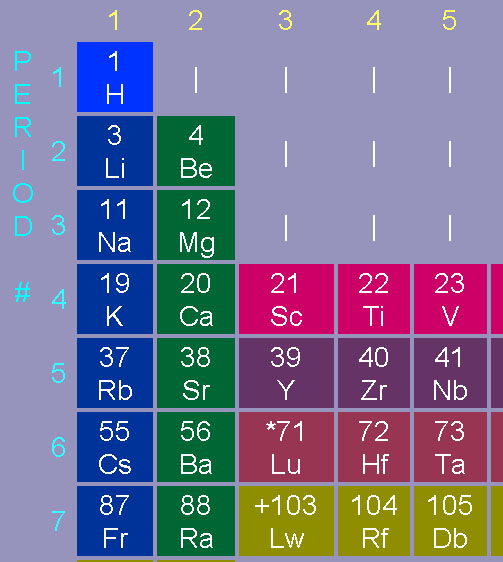
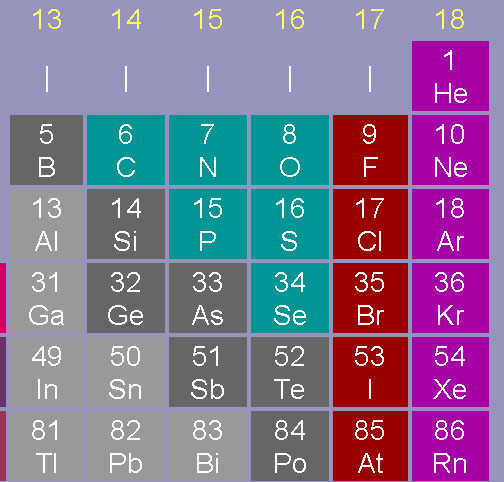
Now find strontium (Sr) in group 2 (alkali earth metals) period 5. Just above strontium is calcium. This is very bad news. Calcium is readily taken up in the body to build teeth and bones. Having similar chemical properties (in same group), strontium will also get absorbed by the body and deposited in bones. Normally, this is not a problem, because naturally occurring strontium (mostly strontium-88) is non-toxic and not radioactive. However, this was strontium-90, which shoots out high speed electrons (beta radiation), which will damage surrounding cells causing bone cancer and other diseases. It's like having miniature hand grenades in the body. Again, the body can't tell the difference between the stable isotope or the unstable (radioactive) isotope.


The effects of the radioactive fallout continues to show up even today because the parents' DNA were affected and these radioactive elements are still present.
Again, the Periodic Table tells us what problems to expect but also suggests ways to trap the radioactive forms of the elements.

As far as classifying the makeup of the universe, we've come a long ways. Our first classification of materials was probably, "Is it edible or not."