#3. The equation E=mc2 can be used to calculate energy given off when mass is converted to energy or how much energy is required to create a certain amount of mass. Mass "m" is kilograms and "c" is speed of light in meters per second. A grain of sand weighs about one millionth of a kilogram (1/1,000,000). So the energy to make that much mas is:
E=
1 kg x(300,000,000m/sec)2
1,000,000
This answer will be in kg·m2·sec-2 (Remember sec-2 is the same as per sec2). These units (kg·m2·sec-2) have conveniently been given the name of joule, which is a common unit for energy. But we want this converted kilowatt hours because we know it costs $0.09 per kilowatt hour. A watt is defined as one joule per second. Below is a spreadsheet style setup of the dimensional analysis needed. Do the arithmetic to solve for the cost to make that much energy. Note that the "/sec" will go to the numerator and get canceled. Actually all units get canceled except dollars. Also note that all conversions have a numerator and denominator that are equal to each other.

Mass |
velocity squared |
conversion |
conversion |
conversion |
conv |
$/kilowatt_hour |
|||||
1 |
kg |
=300000000^2 |
m2·sec-2 |
joule |
watt |
1 |
hour |
kilo |
$0.09 |
= |
$ |
1000000 |
kg·m2·sec-2 |
1 joule/sec |
3600 |
sec |
1000 |
kilowatt·hour |
|||||
#4. Here is a guy who scares his neighbors with his Tesla coils. The sparks are streams of electrons flying off the left donut-shaped metal. Electrons (or electricity) don't usually jump into the air because air resists any electrons trying to move through it.
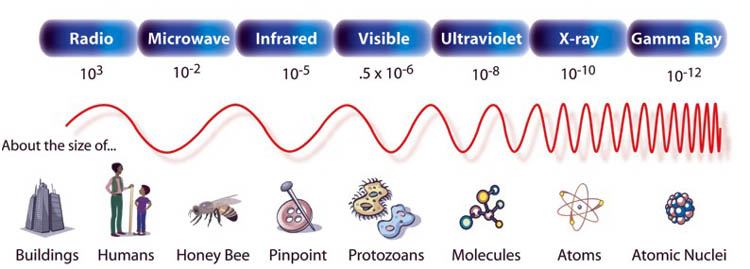
Look at the below illustration of the electromagnetic spectrum. Sparks and lightning produce light (electromagnetic radiation) across most of the spectrum. You have noticed how your radio or TV gets interference from lightning. That's because these produce radio and microwaves. What you don't see is the dangerous ultraviolet light and xrays that sparks create. Our friend here probably doesn't realize the amount of electromagnetic radiation he is exposing himself. I'd say the ultraviolet rays would be the most abundant light. 4a) Do a Web search and report on some dangers to health that ultraviolet light causes. 4b) The below chart has the wavelength of UV light around 10-8, but doesn't say what is the unit of measurement is. What is it?

F= k x q1 x q2
r2
Force will be in newtons (force needed to accelerate a kilogram up to a speed of 1 meter per second and having only one second to do it.) "k" is constant that does the conversion. "q1" is the charge on the proton and "q2" is the charge on the electron. They are equal just opposite. "r2" is the distance (radius) between them squared. Charge is given in coulombs (C) named after a physicist. Below is a spreadsheet way of setting up the calculation.Remember the equal sign "=" causes the spreadsheet to do the calculaton of the scientific notation number. The only unit that doesn't cancel is N (newton). Note the "C-2" goes to the denominator. Do the calculations below to find the force. (For tips on calculating see below the table)

A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
|
| 1 | constant k that converts the units | charge on the proton (q1) |
charge on the electron (q2) |
r2=Distance "r" between proton & electron squared |
conversion to get rid of pico squared |
||||||
| 2 | F= | =8.988*10^9
|
N·m2·C-2 |
=1.602*10^-19 |
C | =-1.602*10^-19 |
C | pico |
pico |
||
| 3 | =70^2 |
(picometers)2 | =10^-12 | =10^-12 | |||||||
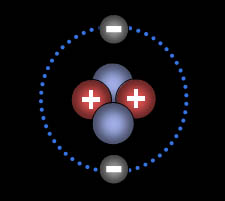
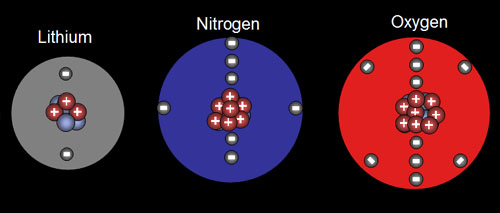
6. From the salad, you are acquainted with the building blocks for the elements at a personal level. Match the atomic particle to the correct sense.
1. Protons
2. Electrons
3. Neutrons.
Senses: Sight, Taste, Pressure (from weight)


10a) What temperature (in Celsius) is necessary to decompose red iron(III) oxide (Fe2O3) which will liberate oxygen? (hint: Do a Google search for: iron oxide melting point. A Wikipedia article should come up. Check it out).
10b) Write the balanced decomposition equation.

11a) What are the three most abundant elements in the Earth's crust?
11b) In the picture snow is on the Earth's crust, so what additional element besides the three mentioned in "11a" is sure to be present?


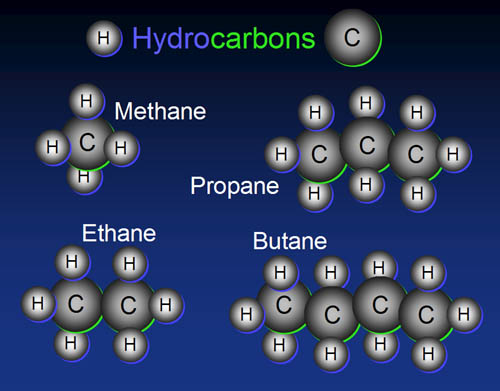
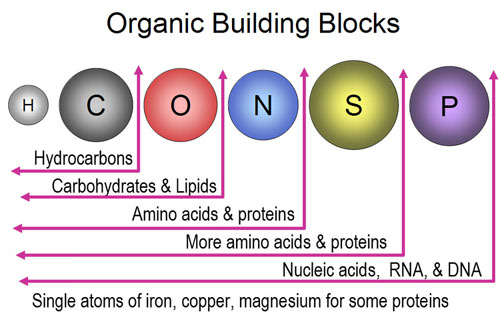
14. Building blocks have to connect to other blocks. In
the picture we see oxygen, hydrogen, and carbon. Looking at the number
of outer electrons each have and the number of vacancies each have...
A) ...what is the maximum of atoms hydrogen can connect to? ...oxygen
can connect to? and carbon can connect to?
B) Which element is the most versatile then?
15b) Some remedies for ant bites use a base to neutralize the formic acid. Baking soda (sodium hydrogen carbonate/NaHCO3) is one of them. What is the missing (???) compound in the below equation for formic acid reacting with baking soda?
HCOOH + NaHCO3 --> HCOONa + H2O + ???


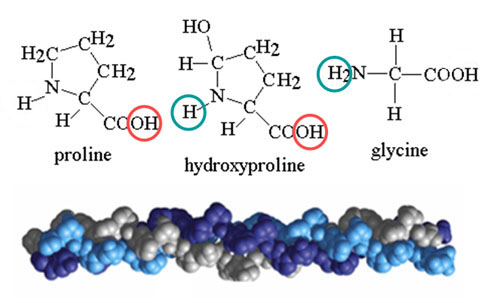
18a) What parts of the body use collagen?
18b) As these 3 amino acids connect, the circled atoms will come off as H2O (Note: on glycine just one of those circled hydrogen atoms come off). What is the molar mass (molecular weight) of the tripeptide that is formed when these 3 amino acids come together?