

| <-CHM151 Home Page | |
| * Apparatus : Bunsen burner -- Calorimeter -- Colorimeter
-- Burette -- Thermometer * Atomic structure : Atom -- Ion -- Electron --
Proton -- Neutron -- Atomic orbital -- Molecular orbital -- Chemical element
-- Valence -- Atomic nucleus -- Isotope * Bonding : Chemical bond -- Ionic
bond -- Covalent bond -- Metallic bond -- Hydrogen bond -- Intermolecular
force -- Dipole * Chemical reaction -- Chemical formula -- Structural formula
-- Mole -- Stoichiometry -- Chemical nomenclature -- Chemical equilibrium
-- Reversible reaction -- Electrophile -- Nucleophile -- Redox * Chemical
techniques : Titration -- Distillation -- Chromatography -- Buffer
solution -- Filtration -- Hydrolysis -- Condensation reaction -- Matter -- Water -- Ammonia -- Benzene -- Phenol * Mixtures
and Solutions: Concentration -- Vapour pressure -- -- Partial
pressure -- solvation * Periodic table : Periodicity -- Group 1 elements
-- Group 2 elements -- Transition metal -- Halogen -- Noble gas -- s block -- d block -- p block -- f block * Properties
: pH -- electronegativity * Structure : Gas -- Liquid -- Molecule -- Solid
-- Isomer -- Allotropy -- Crystal -- Complex -- Ligand -- Chemical
compound -- Stereochemistry |
No Laundry List: In the process of teaching chemistry I was always concerned that the different topics of chemistry seemed disconnected. Without a connection, learning one chemistry topic might not help with a different chemistry topic. However, if there's a connection, then what is learned with one chemistry topic can help in the learning of another. What connects all chemistry topics? At first I didn't know, but I was determined to find it. |
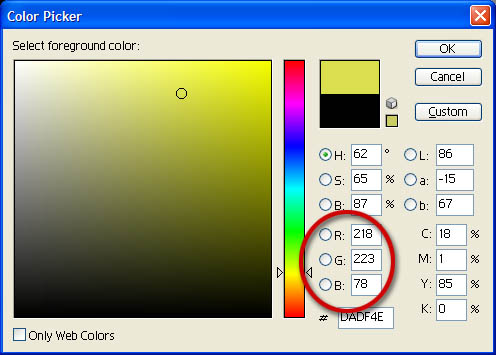 |
ART MEETS SCIENCE: My first clue came from my work with computer graphics. I knew that the 16.7 million colors that a computer screen displays only needs a blend of 3 colors: Red, Green, and Blue. Notice the program allows you to set this particular shade of yellow by typing in values for red, green, and blue. |
|
Reduce 16,700,000 to 3: That simplicity has to be admired. All you can see explained with only 3 colors. So I decided to explain all chemistry topics with only 3 "colors". |
|
 |
Building Blocks: Up to this point I had already emphasized the idea that chemistry is "all about building blocks." Even though building blocks is a good starting point, I knew chemistry is more than just building blocks. I wanted two more parts of chemistry so there would be three, just like the three colors that make up the image on your computer screen or television set. |
 |
FORCE/ENERGY: Some compounds are stable and others explode. So energy and force are another part of chemistry. Energy and force are tightly related. For example, force times distance is one way energy is calculated. Atoms that attract each other with a large amount of force are the same ones that take a lot of energy to pull apart. Instead of making force and energy two separate categories, I felt they are better grouped as one. Now I needed one more category. |
 |
MATH: Measurement is important in chemistry. For example, the difference between a therapeutic dose and a fatal dose is just a matter of magnitude. So math is another critical part of chemistry. This gave me my third "color".
|
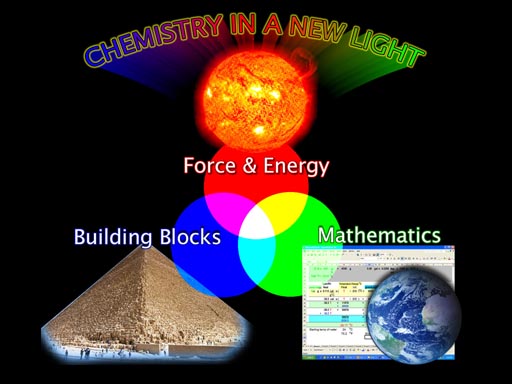 |
TRILOGY ACHIEVED: With these three parts of chemistry identified, I arbitrarily matched them to the three colors that are shown blending to make additional colors. For Force & Energy I chose the Sun to represent that focus. For Building Blocks, a pyramid is used. For mathematics, there's a spreadsheet and the Earth. The Earth was the basis for the metric system, which is heavily used in chemistry. I call this approach, "Chemistry in a New Light" to indicate that this really is a new approach to learning chemistry. |
 |
I use the pyramid to represent the building block aspect of learning chemistry because the great pyramids were built by simple blocks of stone. In other words, something awesome can be made from something quite simple. |
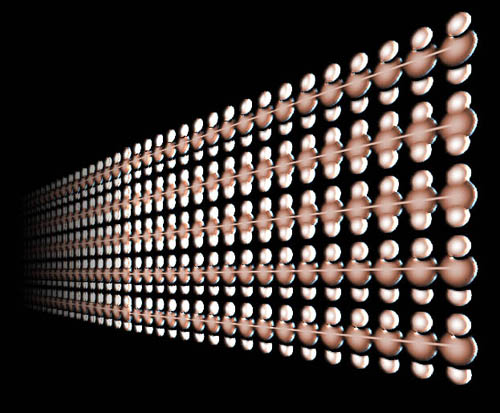 |
Here are chains made up of only carbon and hydrogen atoms. (carbon sits in the middle of two, smaller hydrogen atoms. This is the plastic called polyethylene. Sometimes the chains have half a million carbon atoms with one million hydrogen atoms. At this length the polyethylene is very strong. |
 |
The building block that manufacturers use to synthesize polyethylene plastic is ethylene gas, which is made from 2 carbon atoms and 4 hydrogen atoms. Heat, pressure, or UV light can cause one of these ethylene molecules to attach to another ethylene molecule. When that happens, a chain reaction occurs and within seconds a tank of ethylene gas that can't stop a feather turns into polyethylene plastic that can be used in a vest to stop bullets. It is also tough enough to substitute for ice or be used in knee joint replacement. This is an example of the building blocks of carbon and hydrogen making ethylene, which in turn becomes the building block for polyethylene. So even a molecule that contains one and half million atoms is explained quite simply by its building blocks. |
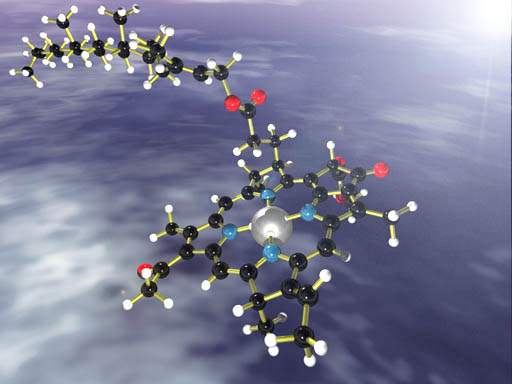 |
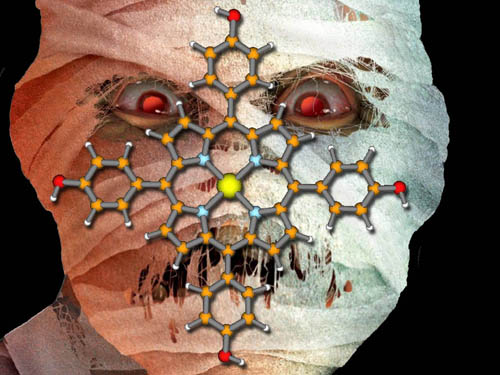
CHLOROPHYLL: This is chlorophyll, the molecule responsible for capturing sunlight and using it for photosynthesis. To understand a complex molecule always look for the simpler building blocks. Identify Atoms: One way to spot building blocks is to look at the different colored spheres. The black ones are carbon atoms. The small white ones are hydrogen atoms. Red is oxygen and blue is nitrogen. The large silver one is magnesium. These five elements are the building blocks of chlorophyll. However, a plant doesn't make chlorophyll using the individual elements. The plant uses a larger building blocks. To spot those blocks look for something that is repeated. |
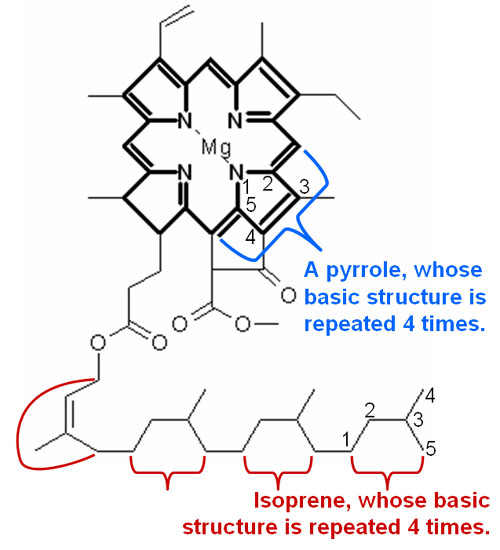 |
Look for Patterns: Here is the chlorophyll molecule drawn again but as a skeletal formula. That means each bend of a line and each end of a line is a carbon atom. Hydrogen is not shown. Oxygen, nitrogen and magnesium are represented by their elemental symbols. At the top, the blue bracket shows a structure that is repeated four times. The nitrogen is part of a 5-sided ring. This is called a pyrrole. Since chlorophyll has four of these rings, this end is called the tetrapyrrole side (tetra means 4). At the bottom we see a repeating pattern of 5 carbon atoms. This building block is called isoprene. Plants and animals use this isoprene building block for all kinds of molecules that it needs. Chlorophyll used 4 of these isoprene building blocks for its "tail". |
 |
In chemistry don't let the large complex molecules scare you. Nature and chemists use simple building blocks, and that's how they make these large complex molecules. Once you discover the building blocks, the whole thing isn't so scary. |
 |
FORCE & ENERGY: Force & Energy is another area of focus when we study a chemistry topic. In the above examples, our focus was on the building blocks. However, force and energy were also involved, we just didn't have to focus on the forces and energies while we were learning about the building blocks. |
 |
Extreme Nuclear Energy: In the case of an atomic bomb, the building blocks are uranium and fast moving neutrons; however, the real focus here would be the forces and energies involved. |
 |
Chemical Energy: The thrust of the two solid rocket boosters is combined with the thrust of the three main engines on the Space Shuttle. The Space Shuttle engines are burning hydrogen with oxygen to make water (steam). The chemical equation is: 2H2+ O2 -> 2H2O The building blocks are simple (hydrogen and oxygen), but here the main focus would be on energy released as the hydrogen is burned. This energy creates the pressure (force) that pushes the rocket forward. |
 |
ELECTRIC CHARGE: The usual force between atoms are electrical, meaning that plus (positive) and minus (negative) charges are at work. Opposite charges attract and like charges repel. This helps explain why some atoms are attracted to other atoms. |
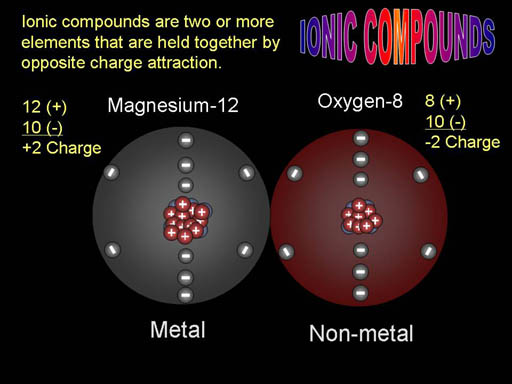 |
Electrical Push & Pull: Ionic compounds are usually formed from a metal combining with a non-metal. In this example, the metal, magnesium, which has 12 protons (particles with + sign) gave away two of its electrons to the non-metal oxygen. When that happens, magnesium is positively charged (protons outnumber the negative electrons) and the oxygen becomes negatively charged. Because they have opposite charges they will attract each other to form a compound called magnesium oxide. Here the building blocks are simply magnesium and oxygen; however, understanding the electrical forces explains why they stick together. |
 |
MAGNETISM: Magnetism is another force that is considered in chemistry under certain conditions. Normally it's not a big factor. Here we see some huge magnets that will be used to change the path of fast flying charged particles, such as a proton (nucleus of hydrogen). Scam Alert: Many bogus products are based on magnets. Notice how large these magnets are just to influence one microscopic particle. Don't be fooled that a regular magnet has the power to heal or improve gas mileage (two common myths). |
 |
GRAVITATIONAL FORCE: Gravitation is another force, but hardly needs considered unless we are talking about the chemistry within a star. In stars the gravitation is so strong that it creates higher pressures within the star. This raises the temperature millions of degrees which allows smaller elements to combine to become larger elements. For example, at 3 billion degrees Celsius, two carbon atoms slam together hard enough that their nuclei fuse to make one atom of magnesium. |
 |
MATH: So far we have discussed building blocks and Force & Energy. We can't forget the mathematics aspect even if you wish to. I chose Earth as part of the icon for mathematics for chemistry. Because measurements were so haphazard, the king of France sent a survey team to measure from Barcelona, Spain to the English Channel. The results was an accurate calculation of the circumference of the Earth. By dividing the circumference by 40 million, they came up with a convenient size that was called a meter. The metric system grew from this measurement. |
 |
MATH EXAMPLE: Remember the energy from burning hydrogen? We know that gasoline and propane burn in vehicles, and that we burn fat and sugar for energy. Could any of these have been used on the Space Shuttle instead of hydrogen? This is where math can help. Hydrogen has 34 food calories of energy per gram. Propane has 12 food calories per gram. Gasoline has 11; Fat has 9, and sugar has 5. Since weight is a big factor for launching something into space, hydrogen gets the most energy for its weight. So the other fuels would work, but their extra weight would cut down severly on the Shuttle's payload. |
   |
THREE FOCUS EXAMPLE:
Force & Energy: Next you can learn the energy and forces involved. You learn that the reaction requires heat to make the oil less viscous and to make the reaction take place. You learn that positive and negative charges on the molecules are the key to the reaction. Also, the forces between the liquids determine what liquids stay together and which separate. You learn that more heat is needed to distill off the methanol. Math: Finally, you focus on the math to answer
these questions: Summary: So by taking the "Chemistry in a New Light Approach" you focus on just one thing at a time, which reduces confusion. Also, by seeing chemistry topics as these three areas of focus, you find out that all chemistry topics have something in common. This helps you apply what was learned earlier to something new. |
Visitors (not just hits) since Aug. 2009