Page 36: Objectives
1. Apply techniques (filtration, evaporation, etc.) to separate the components of mixtures
2. Observe the decomposition of a compound
3. Review physical and chemical properties.
The skills for #1 are quite useful. The allow us to separate out the valuable components from raw materials. For example, it's good to know how to separate out gold flakes and nuggets from a gold and sand mixture. That depends on a knowledge of physical properties such as density and color. Another example, is separating the essential oils from flowers to make perfumes. The essential oils are responsible for the aroma that plants have. If concentrated, they are more valuable and useful.
Through a microscope you can see the difference between sand and salt. Salt crystals are cubic and sand grains are rounded oblong grains. If you had a small enough tweezers and microscope you could separate the salt from the sand one by one. Of course that would be much too tedious. That's why chemists consider other methods. When confronted with this kind of task, it is a good idea to go down the list of physical and chemical properties that the components of the mixture have. You may find some differences that can be used to separate them. Let's look at a few possibilities:

Sand is mostly silica (SiO2=silicon dioxide). If you look up the density of silicon dioxide, you find that the density is 2.2g/cm3. If you look up the density of sodium chloride, you find that it is 2.16g/cm3. Unfortunately, their densities are very close. If the densities were more different, it would be possible to shake the mixture and the more dense substance would settle to the bottom. Earlier you learned that gold had a density of 19.3g/cm3. Compared to sand's density of 2.2g/cm3 gold is about 9 times more dense, so you can see why they can use density to separate gold from sand. But, again, with salt and sand, that's not an option.
Solubility:
This is where we can find a big difference between sodium chloride crystals and sand. Sand does not dissolve in water but salt does. This difference is exploited in this Experiment.
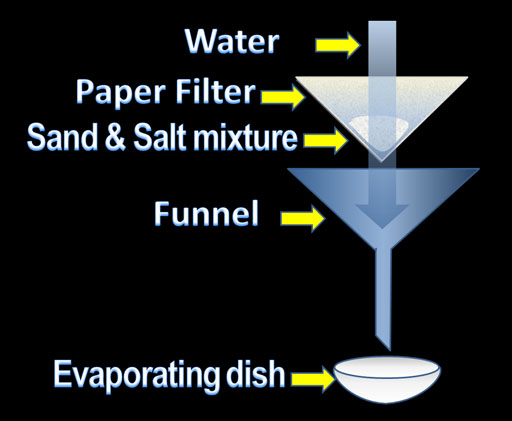
If the task was simply to separate salt from sand, it would be fairly straightforward. We could put the mixture into a paper filter that is set in a funnel. Water can be poured onto the mixture and the water will dissolve the salt carrying it down the funnel and into the evaporating dish. The paper filter will hold back the sand. The evaporating dish will contain a salt solution. If the water is allowed to evaporate, salt will be in the evaporating dish. The sand will be in the paper filter and you have successfully separated the two.
However, this experiment also wants you to determine the percentage (by weight) of the salt and sand. The procedure is basically the same except you have to weigh the initial sand and salt mixture and then the sand and the salt individually after you get them separated.

Page 38 bottom.
B. Decomposition reaction:
As you stand looking at the electrolysis apparatus, which buret contains hydrogen gas, and how do you know?
If sufficient gas has been created, you will see that one of the burets has twice as much gas collected than the other one. Since water is H2O, and both gases are diatomic (H2 & O2), we can assume that the one that is double is the hydrogen.
If the process has just started and you can see the + and - terminals of the power supply, you can say that oxygen is the buret hooked up to the positive terminal.

Solubility is a sliding scale. Some things are very soluble and some things are partially soluble. We know fish has to get oxygen from the water through their gills. So oxygen has to be soluble to some degree. When you look how soluble in water, you find that 8 milligrams of oxygen will dissolve in one liter of water. A liter of water is one million milligrams. So it seems that very little oxygen dissolves in water. Only about 1.5 milligrams of hydrogen dissolves in a liter of water at room temperature.

From before you learned the a physical process doesn't actually change the substance to another substance. Here we are talking about water being changed into two different substances. Also the name "electrolysis" "lysis" means to "break down or break apart" So that's another tip that this is a chemical process.
1. A 10.00 gram sample of a mixture of potassium bromide (KBr) and sand was obtained. Water was added to the mixture to dissolve the KBr as KBr is soluble in water. When the sand was filtered and dried, it was found to weigh 3.595 g. Assume that no sand was lost during the experimental process and that the sand was completely dried. Determine the percentages of sand and KBr in the original sample. Show method.
This is like the earlier experiments. The percentages of KBr and sand are just fractions of the whole weight. The weight of KBr is this fraction of the mixture's weight: 3.595g/10.00g. If we divide 10.00g into 3.595g, we get the decimal fraction of 0.3595 (notice grams cancelled). Percentage is how many hundreds so we can multiply 0.3595 by 100/100 to give us 35.95. The "/100" is replaced with a percent sign (%).
0.3595 x 100 = 35.95 = 35.95%
100 100
If KBr weighed 3.595 grams then sand has to be the remaining weight of 8.405g. (10.00g- 3.595g). Sand's percentage is done the same way. Start with the common fraction 8.405g/10.00g, go to the decimal fraction and then to percent.
Since all measurements have 4 significant digits, then we can leave our answer with 4 significant digits.
This is pretty easy. 50.0% of the 80.0 grams of mixture is is 40.0 grams, and that would be the sand's weight. You can do the rest.
| % NaCl by weight | 1.0 | 3.0 | 4.0 | 6.0 | 8.0 | 10.0 | 11.0 | 12.0 | 13.0 | 14.0 |
| Density (g/mL) at 15C | 1.007 | 1.021 | 1.028 | 1.043 | 1.059 | 1.075 | 1.082 | 1.089 | 1.097 | 1.104 |
What we notice here is not too surprising. We see that as the concentration of the NaCl goes up, the density of the solution gets higher. That makes sense because more salt dissolved will add weight to the water. Pure water is 1.000 g/mL. So all of these are higher than pure water. What is nice about making a graph from this data is that you can find the %NaCl by weight of a solution without going through the whole process of weighing out a sample and drying it to find out the weight of salt. You can simply measure out, let's say 10.0 mL and weigh it. That weight divided by the 10mL gives you the density as grams per mL. Let's say it came out to be 1.067 g/mL. When you find this density on the x axis, you go up to the line and then go to the y axis to see that it corresponds to 9.0% NaCl by weight. So you found out the concentration without all the extra work. Below is the graph I made by using Excel (I cheated). I did have to use PowerPoint to add some extra lines to make it a square grid.
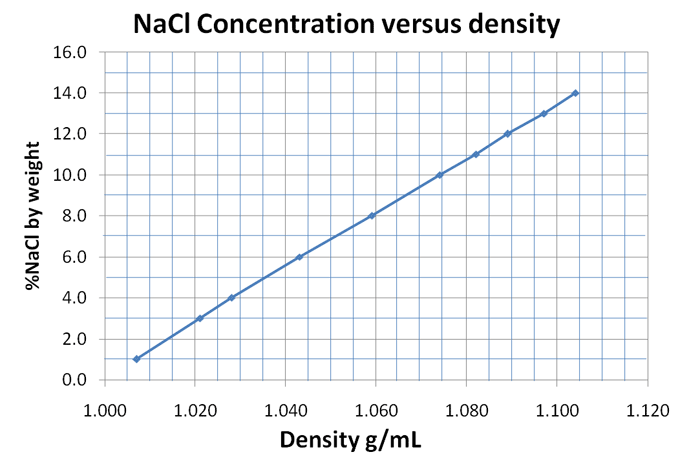
In Excel I typed the data on two rows (I put the densities on the top row). I highlighted the two rows and chose "Insert" followed by chart, then picked the Scatter (X-Y) type of graph. Excel drew lines from each data point to the next, but you should draw one straight line that gets the closest to all points.
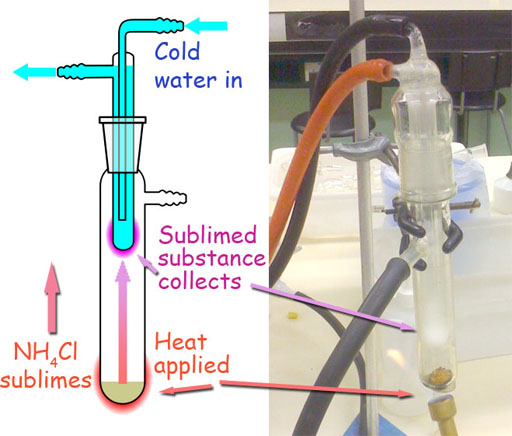
4. A sample is composed of the following substances: ammonium chloride, salt (NaCl), and sand. All are white crystalline solids in appearance but many of their physical properties are different. Using the information on physical properties in the following table, write a procedure of all the steps needed for the separation of the components and describe a method for the percent determination of each component in the sample. Be very specific. Use a separate piece of paper.
| Common Name | Chemical Name | Formula | Sublimation | Water solubility |
| ammonium chloride | ammonium chloride | NH4Cl | sublimates on gentle heating | high |
| salt | sodium chloride | NaCl | none | high |
| sand | silicon dioxide | SiO2 | none | none |
From what you learned in this experiment, you know that you can separate salt from sand using the solubility difference of sand and salt. Here we see that ammonium chloride also dissolves in water. So it can be separated from sand the same way as salt; however, with salt and ammonium chloride together, they both would be dissolved in the water. What makes ammonium chloride different is that it will sublime (go from solid to gas). That means it can be separated from the mixture by heating.