Experiment 10: Properties of Ionic and Covalent Compounds
Page 74: Objectives
1. Describe the characteristics of ionic and covalent compounds, and compare some of their physical and chemical properties.
2. Extract a material from one solvent to another.
3. Study some properties of soaps and detergents.
Exp. 10: Page 76
A. Melting Points of Typical Ionic and covalent substances
| Substance | Formula | Classification (ionic, polar, nonpolar covalent) |
Behavior on Heating |
| sodium chloride | NaCl | ionic | no change |
| sugar | C12H22O11 | polar | Melting, darkening, smoke |
The temperature of a Bunsen burner flame is between 750°C and 950°C. Sodium chloride melts at 800°C. So you might see some melting and you might not. Table sugar is sucrose. It melts at only 186°C and begins to decompose. Even at 160°C it starts decomposing. This decomposition of sucrose is called caramelization. This is how they make caramel candies. About a hundred different compounds are produced when sugar is heated to these its melting point. At higher temperatures sugar will smoke and catch fire.
The bonds of sodium chloride are ionic because sodium completely loses its outer electron to chlorine. That makes sodium +1 and chlorine a -1 charge. Also, when you see a metal combined with a non-metal, you will usually have an ionic compound because metals usually give up their electrons easily, and non-metals usually have a strong attraction to electrons.
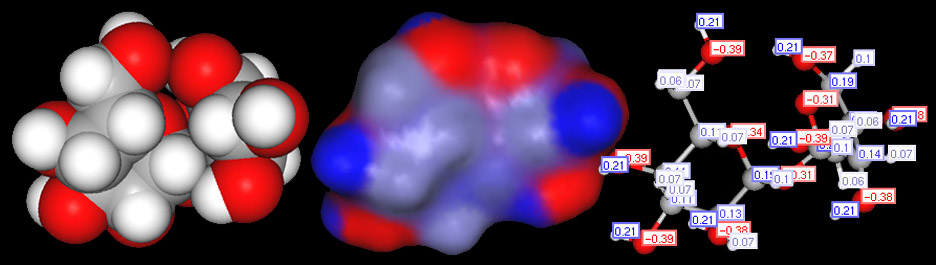
Below is the structural formula for sucrose (left image) and a ball and stick model of sucrose (right image). Carbon, hydrogen, and oxygen are non-metals. When they combine, they use covalent bonds. Since oxygen pulls much harder on electrons than does carbon and hydrogen, the oxygen atoms will have a partial negative charge. With electrons pulled slightly away from the hydrogen and carbon atoms, their protons will be more exposed. So hydrogen and carbon atoms attached to an oxygen atom will be slightly positive. This leaves you with + and - areas on the molecule. Notice that I placed a negative charge by some of the oxygen atoms and a + charge next to the carbon and hydrogen atoms that were bonded to the oxygen. Imagine a line drawn from a + charge to the nearby - charge. That line is like a pole with plus end and a minus end. That why these bonds are called "polar" bonds.

B. Miscibility of Liquids.
Define "miscible"
Go to www.dictionary.com.
Experiment 10: Page 78
1. Classify the following compounds as ionic, polar or nonpolar covalent.
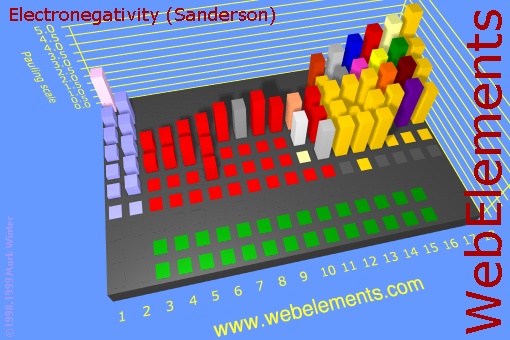
Before we do this section, let's look at the image on the right. This is a 3d view of the Periodic Table of the Elements. The height of the block represents how strong that element pulls on electrons (its electronegativity). You can see that the non-metals, which are up in the upper right corner of the Periodic Table have the highest electronegativity. The metals on the far left (group 1 and 2) have very low electronegativity. The metals in the middle (red blocks) are also low compared to the non-metals on the right. What this means is that when metals combine with non-metals, there is a very uneven pull on the shared electrons. In fact the non-metals usually strip the outer electrons off of the metal leaving the metal a positive charge and the non-metal a negative charge. In other words, the metal and non-metal become ions (charged atoms). So they form ionic bonds and an ionic compound.
Now look at just the blocks of the non-metals. When they combine, they electrons are shared and no ions are formed. So that's a covalent bond. If elements that have the same or similar electronegativities combine, the bond is called non-polar because the electrons are evenly shared and no + and - poles are present. However, if the two non-metals have significantly different electronegativities, then one will pull on the shared electrons more than the other, so there will be some partial positive and partial negative atoms. That makes the bond polar covalent.
1. Classify the following compounds as ionic, polar or nonpolar covalent.CCl4_____________________
Since this is a non-metal combining with non-metal, we have covalent bonding (sharing of electrons). To determine if it is polar or nonpolar, we look at the relative pulling power on electrons (electronegativity) of the atoms. We can try to find carbon and chlorine on the 3d image above, but that's a bit hard. Looking up the electronegativity of chlorine we find the value of "3.16". Carbon's electronegativity is 2.55. If the difference is greater than 0.4 and less than 1.7, then it's usually considered a polar covalent bond. (Less than 0.4 is nonpolar.) Since 3.16-2.55=0.61, it falls in the range of being polar covalent bond. So the carbon-chlorine bonds are polar covalent, with the chlorine atoms being partially negative and the carbon being partially positive. Normally that makes the compound itself a polar covalent compound; however, CCl4 is special because the four chlorines are evenly spaced around the carbon and their polar charges cancel. Notice the +/- poles of the two top chlorine atoms cancel the +/- poles of the bottom two chlorine atoms. So the bonds are polar covalent but the compound is nonpolar covalent.

NaNO3 ___________________
This is a metal (sodium) bonded to a group of non-metals (NO3). So that says ionic compound. When you see NO3 bonded with a metal, you should recognize it as the nitrate (NO3-) ion.
NH4Cl___________________
Even though these are all non-metals, which normally says, "covalent bonds", this is the one case where some of the bonds are ionic. NH3 is ammonia, which has covalent bonds, but a hydrogen ion (H+) is attracted to the nitrogen, turning NH3 into NH4+ ion. The "Cl" here is the chloride ion "Cl-". So the NH4+ ion is attracted to the Cl- ion making an ionic bond and an ionic compound.
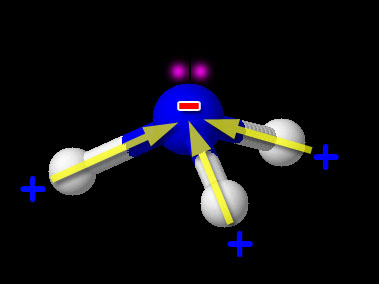
Here we have one non-metal (N) bonded to another non-metal (H). So this is the usual covalent bond. Now we need to see if it is polar or non-polar. We look up the electronegativity of nitrogen and hydrogen. Nitrogen is 3.04 and hydrogen is 2.20. The difference is 3.04-2.20=0.84. This falls in the range of polar covalent bond (between 0.4 and 1.7). Compounds with polar covalent bonds usually make the compound a polar covalent compound unless the polar charges cancel like they did with CCl4. CCl4 was symmetric but NH3 isn't. The image shows NH3 (ammonia). Notice the 3 hydrogen atoms and the +/- poles are all pointing in same direction. So overall the hydrogen end of NH3 is positive and the nitrogen end is negative. So this compound is polar covalent. Regarding the shape of NH3, it's because nitrogen has 5 outer electrons. Three are being shared with the 3 hydrogen atoms. Two unshared electrons on the top push the hydrogen atoms downward.
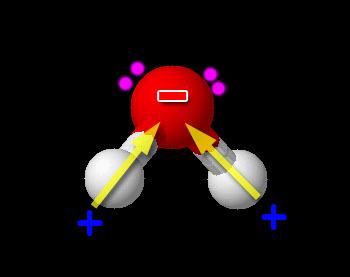
Here we have one non-metal (H) bonded to another non-metal (O). So this is a typical covalent bond. Now we need to see if it is polar or non-polar. We look up the electronegativity of oxygen and hydrogen. Oxygen is 3.44 and hydrogen is 2.20. The difference is 3.44-2.20=1.24. This falls in the range of polar covalent bond (between 0.4 and 1.7). Compounds with polar covalent bonds usually make the compound a polar covalent compound unless the polar charges cancel like they did with CCl4. Water is a bent molecule. The hydrogen atoms and the +/- poles are on the same side, so the poles are reinforced. This makes water very polar. So it is a polar covalent compound. The hydrogen atoms are pushed to one side because there are two pairs of unshared electrons on the other end of the oxygen atom. Oxygen has 6 outer electrons. Two are shared with the two hydrogen atoms. The remaining 4 are the two pairs of unshared electrons.
This is like NaNO3 above. We see a metal (K) bonded with a group of non-metals (SO4). This is your typical ionic compound because the potassium has a charge "K+". SO42- is the negative sulfate ion.
Experiment 10: Page 78
2. Which of the compounds in question 1 above can you be certain are solids at room temperature.
Ionic compounds have ions that attract each other and also the neighboring ions. This increases the strength of holding all the ions together and resisting melting. So the ionic compounds above are the best bet for being solids at room temperature. The next best bet are large polar covalent compounds. In the list above, the ionic compounds and solids are: NaNO3, NH4Cl, and K2SO4. None of the polar covalent compounds are large enough to be solid at room temperature.
3. Refer to section B. Kerosene is a nonpolar compound, water is a polar compound and isopropyl alcohol is a covalent molecule with a polar part and a nonpolar part. How does this explain your observations about the miscibility of each of the pairs tested?
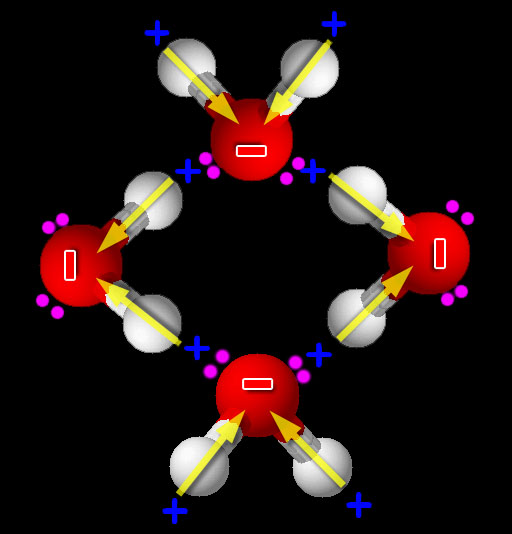
Kerosene and water (I want to do this one first even though it is second in the lab manual):
When things are mixed, the natural tendency is to stay mixed. You know from experience that when things get disorganized and chaotic, it takes energy and effort to bring back some order. So when we have a mixture, there has to be some pretty strong forces to cause these substances to separate and become unmixed (reorganized). Water has that force. We've heard the phrase "water repels oil", but that is not true. What we are seeing is that water has such a strong attraction to other water molecules that the nonpolar molecules like oil or kerosene are squeezed out of the way. (Roll cursor over image to see animation). Because water is polar, the + and - ends of water are attracted to the opposite charges on neighboring water molecules.


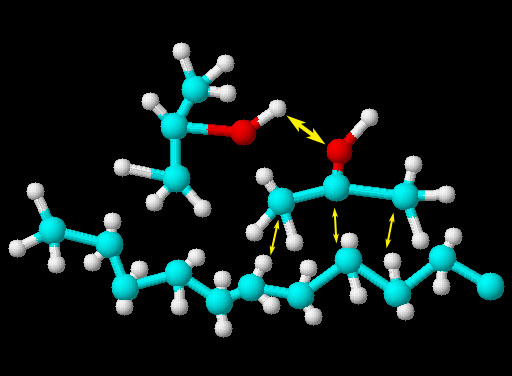
Kerosene and isopropyl alcohol:
Again, when liquids are mixed, the tendency is to stay mixed unless the attraction of on liquid to itself is strong enough to separate itself from the other liquid. The image shows two isopropyl alcohol molecules and one kerosene molecule. Like water isopropyl alcohol has an oxygen atom and a positive hydrogen atom. The larger yellow arrow shows the possible hydrogen bonding of the positive hydrogen to the pair of electrons on the neighboring oxygen. Isopropyl alcohol only has one of these positive hydrogen atoms compared to two on water. Isopropyl alcohol also has a chain of 3 carbon atoms with hydrogens attached. There is a small attraction between these carbon and hydrogen atoms and the carbon and hydrogen atoms of a nearby kerosene molecule. It's called the Van Der Waals force that you will learn about more later. So the reason these two liquids are miscible is that the hydrogen bonding between isopropyl alcohol molecules is not strong enough to overcome the Van Der Waals forces between the 3 carbon chain of isopropyl alcohol and the kerosene molecule.
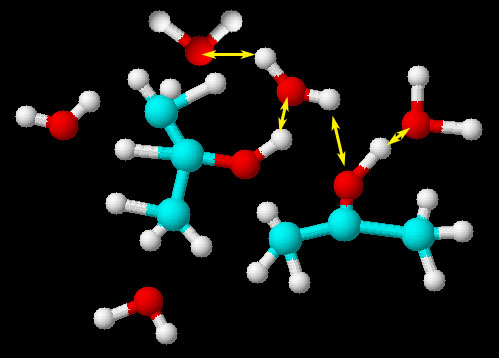
Isopropyl alcohol and water
These two liquids are miscible because they are attracted to each other by hydrogen bonding. In other words water would not push isopropyl alcohol out of its way to get to other water molecules because water is attracted to the OH group on the isopropyl alcohol. In the picture you can see that the positive hydrogen of isopropyl alcohol is attracted to the negative oxygen atoms (lone electron pairs). Likewise, some hydrogen atoms on the water molecules are attracted to the (lone/unshared electron pairs) of the oxygen on isopropyl alcohol. Water molecules will not be attracted to the 3 carbon chains of isopropyl alcohol and for that reason the surface tension of the water will be reduced because not all the water molecules will have something to latch onto. In other words, a mixture of water and isopropyl alcohol dropped onto a something waxy will not form beads. It will spread out on the surface. Note: If an alcohol has much longer carbon chains such as 8 carbons (octanol), the water will have less chance to find the one oxygen on the octanol. So the water molecules will start to bind with each other more and the two liquids will separate. There will be a few water molecules attached to the OH of the octanol, but most of the water will separate into a water layer.
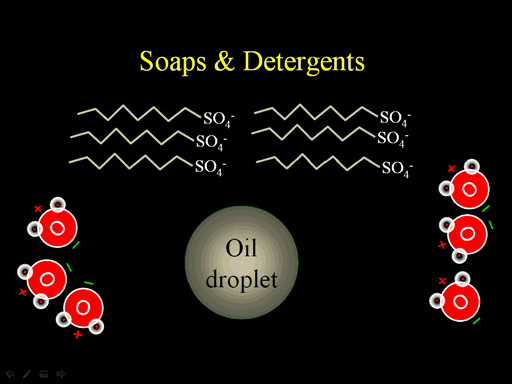
4. In general, detergents are long molecules with a polar end and a nonpolar end. How does this explain their ability to remove oily stains from clothing in your washing machine.
In the picture you see an oil drop. Water is not attracted to the oil. Water is only attracted to things with a charge. Notice the soap molecule. The zigzag represents a long chain of carbon and hydrogen atoms (a nonpolar hydrocarbon chain). Water has no attraction to that chain or the oil droplet. However, if the long hydrocarbon chain (that is the nonpolar end of soap) contacts the oil droplet, it will mix with the oil. Remember there is some Van Der Waals attraction between these chains. The polar end (SO4- end) will be attracted to the water and not go into the oil. With several of these SO4 ends sticking out, water will be attracted to the oil drop and keep it suspended in the water. So with the water goes out with the rinse, the oil goes with it. (Roll cursor over the image to see animation)
The I-I bond is covalent, more specifically nonpolar covalent. Since the iodine left the water layer and moved into the kerosene layer, it shows that water had very little attraction to it. In that case it must be a nonpolar covalent compound. Note: Since two iodine atoms would share their electrons evenly (equal electronegativity), we would expect a nonpolar bond.
6. When methanol (CH3OH) is combined with hydrochloric acid, possible products are methyl chloride (CH3Cl) and water. Methyl chloride is a colorless gas at room temperature, and it is insoluble in water. Would you conclude methyl chloride is ionic or covalent?
You answer this one.
Write a balanced equation for this reaction:
CH3OH(l) + HCl(aq) --> CH3Cl(g) + HOH(l)
Experiment 10: Page 79
By a process of selection and elimination, choose from the list of solvents below the one that would best dissolve each substance given in the table.
solvents: a.) H2O b.) C7H16 c.) none listed
| substance | best solvent | substance | best solvent |
| CCl4 | C7H16 | sugar(C22H12O11) | ??? |
| ethanol (C3H7OH) | ??? | a gasoline component (C8H18) | ??? |
| road tar (C38H78) | ??? | HF | ??? |
A good rule is that like dissolves like. Earlier in this lab I explained that CCl4 is a nonpolar covalent compound. Being nonpolar liquid, you use another nonpolar liquid to dissolve it. That would be the hydrocarbon heptane (C7H16). You know from experience that sugar dissolves in water, so you would pick H2O. You now know the reason is that sugar has several OH groups that allow for hydrogen bonding. So water can pull it apart. Ethanol that we drink is always diluted with water, so we know if must dissolve in water. Ethanol also has an OH group that water will latch onto. Gasoline component octane (C8H18) is almost the same as heptane (C7H16). Since like dissolve like, these will be miscible. Road tar is also a hydrocarbon like heptane. HF is polar because of the big difference in the electronegativity of hydrogen (2.20) versus fluorine (3.98).
Reaction Review
Complete the word equations and write balanced formula equations for the following reactions. Classify each of the reactions as combination, decomposition, single replacement, double replacement, combustion reaction.
1. Aluminum + zinc sulfate --> Zinc + aluminum sulfate
Formula Equation:
2Al(s) + 3ZnSO4(aq) --> 3Zn(s) + ???(aq)
2. Calcium oxide + water --> calcium hydroxide
Formula Equation:
CaO(aq) + H2O(l) --> ???(aq)
3. Propane gas + oxygen --> carbon dioxide and water
Formula Equation:
C3H8(g) + ?O2(g) --> ?CO2(g) + ?H2O(g)
Formula Equation:
2KOH(aq) + H2SO4(aq) --> ???(aq) + 2HOH(l)
Formula Equation:
?Al(s) + ?HCl(aq) --> ?AlCl3(aq) + ?H2(g)