
The chore of counting is simplified by weighing and then calculating the number.
This trick does not work with mixtures. For example, you can't weigh all these coins together and be able to count them. You have to separate them first. The same thing is true with chemicals. It must be pure, or at least you need to know what percent the various components are.

Problem 1:
A standard roll of nickels contain 40 nickels with a value of $2. Nickels weigh 5.00 grams. The paper roll weighs 1 gram. What should a roll of nickels weigh?
Problem 2:
A stack of nickels weigh 326 grams. How many nickels are present?
Problem 3:
For black tattoos, they sometimes use iron (II) oxide with formula of FeO. To make it you need equal numbers of iron and oxygen atoms. 1 mole of iron atoms and 1 mole of oxygen atoms would be one option. How many grams of each would that be?
Problem 4: What would be the total weight of the FeO pigment created if we used 1 mole each of Fe and O?
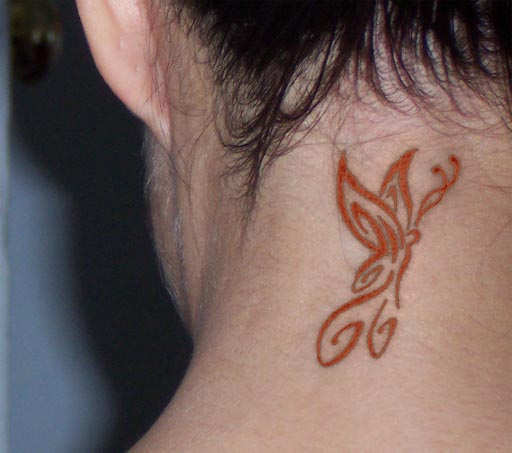
If this lady were to bleach her hair, she may cause the
iron (II) oxide in the tattoo to become iron (III) oxide, which is reddish.
The butterfly now looks like flames. Remember the "(III)" means
the iron has 3+ charge. If oxygen has 2- charge, the formula has to be
Fe2O3 in order for the charges to balance
2x(+3)=3x(-2).
Problem 5: To make 10 molecules of the iron (III) oxide pigment (Fe2O3), how many atoms of iron is needed and how many atoms of oxygen are needed?

Here's a specimen of hematite, which is iron (III) oxide. If we wanted to make a pound (454g.) of hematite, how many grams of iron is needed?
The information here is all in grams, but the strategy we just learned is that by finding the number of Fe2O3 molecules, we can easily get the number of iron atoms. So the first step is to convert 454 grams of Fe2O3 into moles of Fe2O3. To do that means we have to know what one mole of Fe2O3 weighs. The Periodic Table is where we look. The table in the textbook shows iron as 55.85 grams per mole and oxygen as 16.00 grams per mole. For Fe2O3, we see there will be 2 iron atoms and 3 oxygen atoms
Fe2= 55.85 g. x 2 = 111.7 grams
O3 = 16.00 x 3 =
48.00 grams
Added together = 159.7 grams for one mole of
Fe2O3.
454 grams Fe2O3 x 1 mole Fe2O3 = 2.843 moles Fe2O3
159.7 grams Fe2O3
At this point we have the number of Fe2O3 molecules. It's easy to see the number of iron atoms, because it's just double that of the Fe2O3 molecules. So the number of iron atoms is 2.843 moles x 2 = 5.686 moles. So to make up a pound of the hematite we need 5.686 moles of iron. We can't count this many iron atoms, but we can change moles to grams, which is just the reverse of what we just did. Again the Periodic Table says a mole of iron weighs 55.85 grams. Since we have 5.686 moles, we can do this multiplication:
5.686 moles x 55.85 grams Fe = 317.6 grams of Fe.
1 mole Fe
So to make 454 grams of hematite, we need 317.6 grams of iron. The remaining grams (454-317.6=136.4) must be oxygen.


Problem 7: If you wanted to make one pound of magnetite, how many grams of iron would be required?
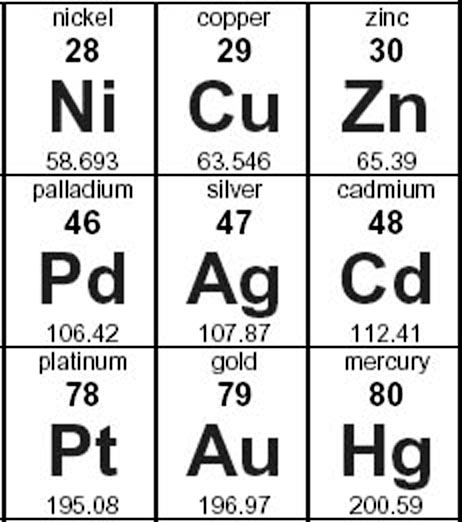
Drill and Practice: I'm not that crazy about drill & practice, but it's important to do these kind of calculations almost automatically.
Here's a section of the Periodic Table. Let's say we have 5.00 grams of each metal (same weight as a nickel coin), how many moles of each do we have?
I'll do copper (round to 3 sig figs):
5.00 g. x 1 mole = 0.078683 = 0.0787 moles
63.546 g.
Problem 8: Do the remaining metals on the chart.

Blood test results:
There's a big push to get blood test results reported using an international standard. The organization that has promoted metrics and other standards is called the International System of Units (or SI from the French, Le Système International d'Unités). For example, the blood tests results in the United States are shown differently than what is displayed in other countries. That can lead to confusion and errors.
For example, the normal level of calcium in the blood is 9 to 10.5 milligrams per deciliter (tenth of a liter). In the SI units, they want it in millimoles per liter. (millimole is a thousandth of mole) To do these conversions one needs to convert grams to moles, just like the above problems were doing. I'll do one problem, and you do the rest. Right now we won't deal with the volume (deciliter versus liter), we'll save that for a later chapter. For now we are just converting grams to moles.
Calcium 9.00mg-10.5mg is normal: Periodic Table shows 40.08 grams per mole. Notice that the "m" in milligrams does not cancel (just grams), so we end up in millimoles, which is what we want.
9.00 mg x 1 mole = 0.225 millimoles (mmol)
40.08 g.
10.5 mg x 1 mole = 0.262 millimoles (mmol)
40.08 g.
So the normal range in the new SI units is 0.225-0.262 mmol.
Normal range for Iron is 60-150 mg.
Normal range for zinc is
75-120 mg.
Normal range for lead is less than 40mg.
Problem 9: Report the normal range of these metals in millimoles.
