
After doing tutorial on "Language of Chemistry", Read Chapter 6.
11th Edition: Pages 103 to 121
12th Edition: Pages 103 to 121
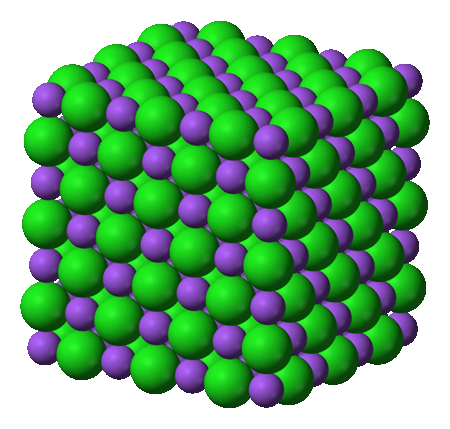
Problem 1:
11th Edition, page 122, Question 13
12th Edition, page 122, Question 5
Write the systematic names for the following:
a) Laughing gas (N2O)
b) Calcite (CaCO3)
c) Alumina (Al2O3)
d) Galena (PbS)
e) Muriatic Acid [HCl(aq)]
f) Table salt (NaCl)
Bonus question: The image on the left could represent which two compounds
above?
Problem 2:
11th Edition, page 122, Question 17
12th Edition, page 122, Question 11
Write the formulas for these binary compounds, all of which are composed
of nonmetals.
a) carbon monoxide
b) sulfur trioxide
c) carbon tetrabromide
d) phosphorus trichloride
e) nitrogen dioxide
f) dinitrogen pentoxide
g) iodine monobromide
h) silicon tetrachloride
i) phosphorus pentaiodide
j) diboron trioxide
Bonus question: How healthy do you think the above compounds are?


Problem 3:
11th Edition, page 122, Question 18
12th Edition, page 122, Question 13
Name these binary compounds; all of which are composed of non-metals.
a) CO2
b) N2O
c) PCl3
d) CCl4
e) SO2
f) N2O4
g) P2O5
h) OF2
i) NF3
j) CS2
Bonus question: Two of these compounds are used for the "cold"
fire magic trick. They are CCl4 and CS2. Are these
compounds a gas, liquid, or solid at room temperature?