Page 64: Objectives
1. Review general pattern for double replacement reactions
2. Predict if a reaction will occur based on a few simple rules.
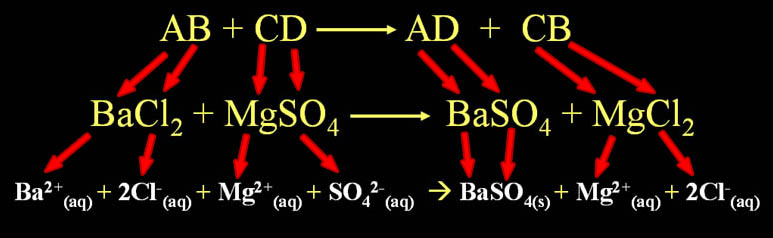
Double Replacement Pattern: AB + CD --> AD + CB
(Two compounds exchange ions to form two new compounds)
Note: The letters are just letters that represent any ion. The "B" is not boron and "C" is not carbon.
Discussion: A water solution of .... (The discussion in the lab manual is good. Below I will give a real world example of how one might use a double replacement reaction).
Experiment 9: Page 65
2. An unstable compound that breaks down to give a gas and water.
Carbonic acid: H2CO3 --> CO2(g) + H2O(l)
You know this reaction well. This is the reaction that gives your sodas the fizz.
Sulfurous acid: H2SO3 --> SO2(g) + H2O(l)
The reverse of this reaction is SO2 mixing with water (rain) to make acid rain (H2SO3).
Ammonium hydroxide NH4OH --> NH3(g) + H2O(l)
When you buy ammonia cleaner, you are actually buying ammonium hydroxide in water. However, the smell of ammonia coming from the container is because some of the ammonium hydroxide is decomposing into ammonia gas as shown in this reaction.
What ions have to be brought together to give H2SO3?
For acids, one ion is always "H+". Here we have two "H+" with a total 2+ charge. The other ion must be negative 2 (2-) charge. It is SO32-. This is called the sulfite ion. What this means is if sulfite ions (SO32-) come in contact with an acid (H+), sulfurous acid will form, which then may proceed to decompose into SO3 gas and H2O.
What ions have to be brought together to give NH4OH?
Here you should recognize two polyatomic ions, NH4+ and OH-. If one solution has NH4+ ions and another has OH- ions, bringing them together will form NH4OH, which can decompose into the gas NH3 and water.
All of these reactions are reversible, but if the gas escapes, then reaction can't go in reverse and we get new compounds forming. For example, here is an example related to sulfurous acid:
Na2CO3 + 2HCl --> 2NaCl + H2CO3
This is followed by the carbonic acid decomposing:
H2CO3 --> H2O + CO2(g)
The final products in the water will be salt (NaCl) and a little extra water. The CO2 will have escaped.
So this is how washing soda (Na2CO3) can neutralize hydrochloric acid.
3. Molecular or unionized compound that stays in solution.
(See description in lab manual)
Write word and formula equations for sodium hydroxide, NaOH(aq) and phosphoric acid, H3PO4(aq): WORD EQUATION:
Sodium hydroxide and phosphoric acid --> sodium phosphate and water
FORMULA EQUATION:
First write the unbalanced equation:
???(aq) + H3PO4(aq) --> Na3PO4(aq) + ???(l)
Note: We had to use 3 Na because PO4 has a negative 3 charge. It takes 3 Na+ to balance the charge.
To balance the equation notice that there are 3 Na in the products so we need to start with 3.
3NaOH(aq)
+ H3PO4(aq) --> Na3PO4(aq) + H2O(l)
The sodiums (Na) balance but the hydrogen atoms don't. This is why it is clearer if we write H2O as HOH. That way we can keep better track of the H+ and the OH-.
3NaOH(aq)
+ H3PO4(aq) --> Na3PO4(aq) + 3HOH(l)
AB + CD --> AD + CB
Now we can see that there are 3 OH ions in the reactants and 3 in the products. We can also see the H+ came from the phosphoric acid and ended up in the water. Writing HOH also helps us see the double replacement pattern.
Experiment 9: Page 70
1. sodium chloride and silver nitrate
Observation:
Hopefully, you saw a white precipitate form.
Formula equation:
NaCl(aq) + AgNO3(aq) --> NaNO3(aq) + ???(s)
Why does this double displacement (replacement) reaction occur?
A short answer to this question is, "It formed a precipitate." A little longer answer is , "It occurred because silver chloride is not soluble in water so it formed a precipitate."
2. sodium chloride and ammonium nitrate.
Observation:
You probably saw no signs of a reaction.
Formula equation:
NaCl(aq) + ??? --> No reaction
Why does this double displacement (replacement) reaction occur?
It doesn't occur because all ammonium compounds are soluble and all nitrate compounds are soluble.
Observation:
You probably saw a lot of bubbles.
Formula equation:
Na2CO3(aq)+HCl(aq) --> 2NaCl(aq) + H2CO3
V
H2CO3 --> CO2(g) + H2O(l)
Why does this double displacement (replacement) reaction occur?
The formation of an unstable compound that breaks down into a gas and water. In this case the unstable compound is carbonic acid. The gas is CO2. Also, you know that a warmer solution will cause the carbonic acid to decompose more readily. Under high pressure and cold temperatures, there may be no decomposition of carbonic acid.
Observation:
Hopefully, you noticed some heat was generated. If these were concentrated solutions of sodium hydroxide and hydrochloric acid, the heat is enough to boil the water and spray solution everywhere. Weak solutions will just produce some heat.
Formula equation:
NaOH(aq) +???(aq) --> 2NaCl(aq) + ???(l)
Why does this double displacement (replacement) reaction occur?
The formation of an unionized compound that remains in solution. In this case it is the formation of water from H+ and OH-. Water does not dissociate back into H+ and OH-. So the reaction will not go backwards.
Experiment 7: Page 71
5. barium chloride and sulfuric acid:
Observation:
Hopefully, you noticed a white precipitate.
Formula equation:
BaCl2(aq) + H2SO4(aq) --> ???(s) + HCl(aq)
Why does this double displacement (replacement) reaction occur?
See why AgCl reaction shown above occurred.
6. barium chloride and silver nitrate:
Observation:
Hopefully, you noticed a white precipitate.
Formula equation:
BaCl2(aq) + AgNO3(aq) --> Ba(NO3)2(aq) + ???(s)
Why does this double displacement (replacement) reaction occur?
See why AgCl reaction occurred in the earlier problem.
7. ammonium hydroxide and sulfuric acid:
Observation:
You probably noticed some heat.
Formula equation:
NH4OH(aq) + H2SO4(aq) --> NH4SO4(aq) + HOH(l)
Why does this double displacement (replacement) reaction occur?
8. sodium chloride and zinc nitrate
Observation:
No signs of a reaction.
Formula equation:
NaCl(aq) + Zn(NO3)2 --> No reaction
Why does this double displacement (replacement) reaction occur?
It doesn't occur because sodium nitrate is soluble and zinc chloride is soluble. No unstable compounds are formed and no unionized compounds are formed.
9. sodium carbonate and calcium chloride:
Observation:
Hopefully, you noticed a white precipitate.
Formula equation:
Na2CO3(aq) + CaCl2(aq) --> ???(aq) + CaCO3(s)
Why does this double displacement (replacement) reaction occur?
10. iron(III) chloride and ammonium hydroxide:
Observation:
You may have seen a yellow precipitate.
Formula equation:
FeCl3(aq) + NH4OH(aq) --> Fe(OH)3(s) + NH4Cl(aq)
Why does this double displacement (replacement) reaction occur?
11. A small scoop of baking soda (NaHCO3) also called sodium hydrogen carbonate in a few mL of vinegar (dilute acetic acid, HC2H3O2):
Observation:
You probably saw bubbles formed.
Formula equation:
NaHCO3(aq)+HC2H3O2(aq) --> NaC2H3O2(aq)+H2CO3
V
H2CO3 --> CO2(g) + H2O(l)
Why does this double displacement (replacement) reaction occur?
Problems
A. Identify the ions present in each reaction and write formulas for the reactants. Then write the potential products on the product side. Circle each product that removes ions from the solution and note how: precipitate, gas, or molecule. If the reaction occurs, balance the equation. If no ions are removed (all are aqueous), then write NR.
1. Lead(II) nitrate and potassium chloride
Pb(NO3)2(aq) + 2KCl(aq) --> PbCl2(s) + 2KNO3(aq)
[removed as precipitate] (red indicates circled)
We know lead forms a precipitate because the solubility table shows that Pb2+ forms a solid with Cl-. The solubility rules also indicate that.
2. Phosphoric acid and potassium chloride.
H3PO4(aq) + KCl(aq) --> NR
The solubility table shows that K+ and PO43- stay aqueous (are soluble). H+ and Cl- are also shown to remain aqueous. If all combinations are soluble (stays aqueous), nothing happens.
3.Barium chloride and sodium phosphate.
?BaCl2(aq) + ?Na3PO4(aq) --> Ba3(PO4)2(s) + ???(aq)
[removed as precipitate] (red indicates that this compound should be circled in answer)
The solubility table shows that Ba2+ mixed with PO43- forms a solid (precipitate). This reaction was a little harder to balance because phosphate is negative 3 and barium is positive 2.
4.Chalk (calcium carbonate) and vinegar (acetic acid solution).
CaCO3(aq)+?HC2H3O2(aq) --> Ca(C2H3O2)2(aq)+ ???
V
??? --> CO2(g) + H2O(l)
[removed as gas] (red indicates circled)
Note: CaCO3(s) (chalk) starts off as a solid, but some of it dissolves to make CaCO3(aq). The acid from the acetic acid combines with the carbonate to make carbonic acid. Carbonic acid is unstable and will decompose to CO2 and water. Note that calcium is 2+ and acetate is 1-, so you have to have two of the acetate ions to balance with the calcium.
5. Sodium sulfite and sulfuric acid.
Na2SO3(aq) + ???(aq) --> Na2SO4(s) + H2SO3
H2SO3 --> ???(g) + H2O(l)
[removed as a gas] (red indicates that this compound should be circled in answer)
Sulfurous acid is unstable and will decompose into SO2 gas and water.
6.Sodium carbonate and sulfuric acid.
???(aq) +H2SO4(aq) --> Na2SO4(aq) + H2CO3
B. Write word and formula equations for reactions given. FORMULAS AND NAMES MUST BE CORRECT. Label each equation as DR (double replacement) or SR (single replacement).
Complete the word equation and write a formula equation for each reaction:
1. Magnesium oxide + __________ --> Water + Magnesium chloride
To know what compound is missing we should write out the formula equation with what we've got:
MgO + ? --> H2O + MgCl2
It appears that our products now have hydrogen (H) and chlorine Cl). So what is the missing acid?
FORMULA EQUATION:
MgO(aq) + ???(aq) --> H2O(l) + MgCl2(aq) [DR]
It's double replacement because Mg ends up with the chloride ion and the H+ ends up with the oxygen. So there was a swap of the ions.
Just like #1, the products have hydrogen and chlorine which is not listed in the reactants.
FORMULA EQUATION:
Mg(s) + HCl(aq) --> H2(g) + ???(aq) [SR]
This is a single replacement because one reactant (Mg) was not combined with anything. It replaced the hydrogen on the hydrochloric acid.
To know what compound is missing we should write out the formula equation with what we've got:
Zn(OH)2(aq) + H2SO4(aq) --> ? + ?
This is a typical double replacement neutralizing reaction where H+ combines with OH- to form water. So water is one of the products. That means zinc remains to combine with the sulfate. What are the missing product names?
.
FORMULA EQUATION:
Zn(OH)2(aq) + H2SO4(aq) --> ???(l) + ZnSO4(aq)
Like #2 above, many metals in contact with a strong acid like hydrochloric acid give its electrons to the H+ (acid). The chlorine from the hydrochloric acid then combines with the metal.
FORMULA EQUATION:
Sn(s) + HCl(aq) --> ???(g) + SnCl2(aq)
5. Copper (II) hydroxide + ________ --> Copper (II) sulfate + water
To know what compound is missing we should write out the formula equation and see what we've got:
Cu(OH)2 + ? --> CuSO4 + HOH
From this we can see that there is sulfate (SO4) on the product side but not the reactant side. The negative sulfate ion would not be alone. There must be a positive ion that came with it. We don't see any new positive metal ion on the product side, so the positive ion with the sulfate must be H+. So what is the name of the missing reactant?
FORMULA EQUATION:
Cu(OH)2(aq) + ???(aq) --> CuSO4(aq) + HOH
This is another acid reacting with a base (OH) to form water. What kind of reaction is that?