

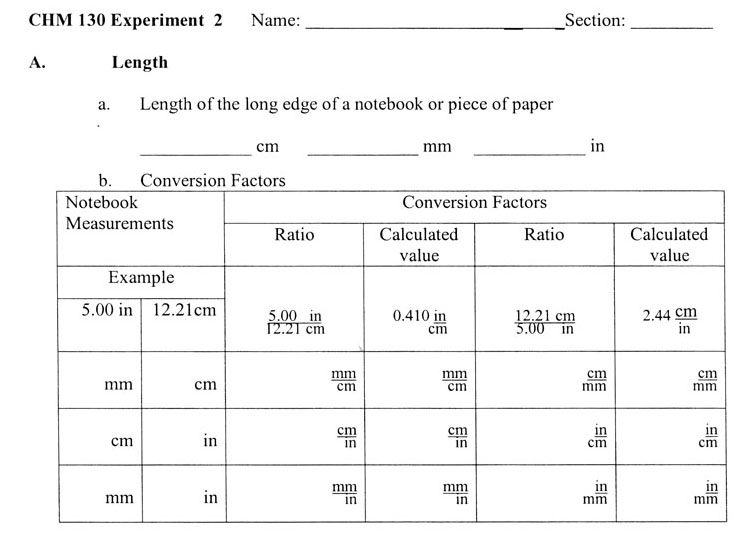
A. Length
2. You will calculate your own conversion factors from your measurements.
You will measure your notebook in inches, millimeters, and centimeters. When you measure the notebook edge's length in inches, measure to the nearest sixteenth of an inch. Let's say your notebook paper is closer to 10 7/8 inches than 10 15/16. So you call it 10 7/8 inches. Turn the common fraction (7/8) into a decimal fraction, which is 0.875 inches. So the length in inches is 10.875 inches. You might think you've got 5 significant digits; however, you measured to the nearest sixteenth of an inch (0.0625). So you see the digits after the "10.8" are not significant because the accuracy of 0.0625 will alter them. However, for calculation purposes just leave it as 10.875 inches, but realize it's only 3 significant figures.
Now measure in centimeters. Let's say it is 27.4 centimeters. That means you measured to the nearest millimeter. Here you also have 3 significant digits (figures). Place your data on the data sheet on page 18. Data sheet is shown just after this box.
A conversion factor is made from an equality. In our case the measurements are of the same edge of paper, so 10.875 inches = 27.4 centimeters. Conversion involves canceling one unit. So we can set up this way if we want cancel out centimeters.
Known centimeters x 10.875 inches = same length in inches
27.4 centimeters
The fraction bar can be interpreted as divided by, per, for each, or for every. If we wanted to say inches for each centimeter, we can reduce the fraction this way:
10.875 inches ÷ 27.4 = 0.39689 inches
27.4 cm ÷ 27.4 1 cm
We need to round our answer to 3 significant digits. So we now say 0.397 inches per centimeter or (0.397 inches for each centimeter). That's easier to use or remember than 10.875 inches per 27.4 centimeters.
When you measure centimeters and millimeters, there shouldn't be any difference in the numbers except for the decimal place. The reason is that when measuring centimeters, you use the millimeter divisions to get a tenth of a centimeter. In other words in our example above, your measurement of 27.4 centimeters should be 274 millimeters when you count the millimeters. So the conversion factors for cm and mm will be a factor of 10. For example,
10mm or 0.1cm
cm mm


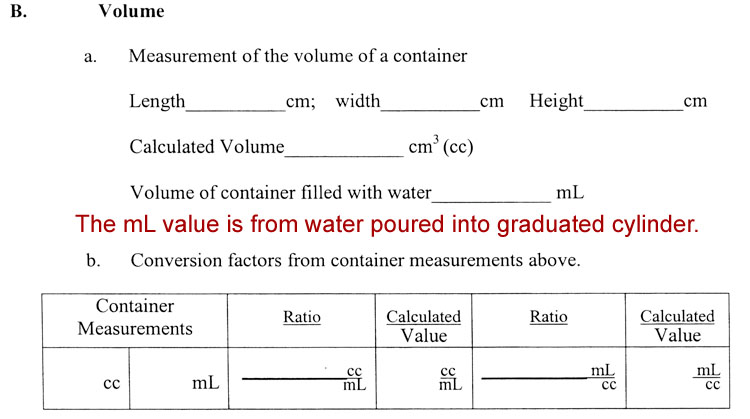
B. Volume: 3. Calculate the conversion factors between cm3 and mL from your measurements.
Normally, we assume the theoretical (defined) value of 1mL=1cc, with conversion factors written as
1mL or 1cc
1cc 1mL
However, it's good to prove that to yourself like you did in Experiment 1.
Let's say the measurements of the box were 8.5cm x 5.3cm x 3.4cm. That multiplies out to be 157.17cm3. Notice the answer has 5 significant digits, but we are only justified in using two because our measurements were had only two significant figures. In this case, when you fill the container with water and then pour the water into a 100mL graduated cylinder, it's going to be more than one full graduated cylinder. Just fill it somewhere between 80 and 100mL and read the exact amount and write it down. Empty the graduated cylinder and pour the rest of the water into it. Add the to volumes together. Let's say the volume added up to 159.4 mL. See data page below for how to record your volume data and make conversion factors.
157.17cc
159.4 mL
The Calculated Value (cc per 1 mL) is found by dividing both numerator and denominator by 159.4, which gives us 0.986cc per mL (using 3 significant digits).
0.986cc
mL
The reverse ratio would be the same figures but inverted:
159.4 ml
157.17cc
The Calculated Value (mL per 1 cc) is found by dividing both by 157.17, to give 1.01mL per cc (written with 3 significant digits).
1.01mL
cc

Percent Composition
In chemistry understanding the percent composition is important for various reasons. If the task is mining, then knowing the percent composition of what you are after lets you know if you will make a profit. If the sample is medicine, then the percent composition lets you know the correct dosage. In the making of salt, the percent composition will let you know how much salt you will retrieve.

You are going to be given some salt water. The lab manual says it has "unknown composition", however, the instructor and lab technician know the composition. It is just unknown to you. Your task is to find the percent composition and see if it matches the known value. The formula above indicates that if you need two quantities: grams of NaCl (salt) and the grams of the solution that holds that salt. So that's just two weights that you need. However, in chemistry you never weigh the grams of a powder (salt) or the grams of a solution directly on the balance. These have to be in some kind of container. So that means you usually have to know the weight of the container. In this lab, the solution is placed in an evaporating dish. So you need the weight of that so you can subtract it from the weight of the dish plus the solution.

It says to put in about 3 mL of the salt solution. Because you are going to weigh it, you don't need an exact volume. If the task was to find %w/v (weight per volume), then you would have found the exact volume. But since we are finding weight (grams) of salt per weight (grams) of solution, we don't have to measure volume exactly. The 3 mL is just an amount that gives you enough to weigh without being so much that it takes a long time to evaporate.
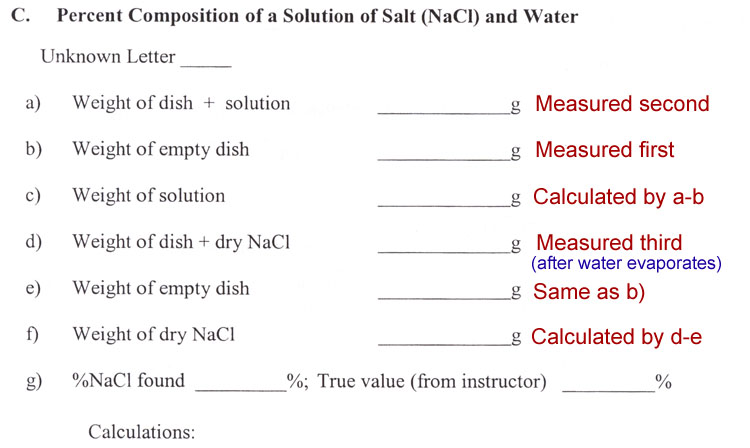
g) %NaCl found
A percent is a fraction, more specifically percent means per hundred ( or hundredths). We know the weight of the NaCl has to be a fraction of the solution's weight. If NaCl was 1 gram and the solution was 4 grams, we'd say NaCl was 1/4 of the weight of the solution. (1gram NaCl per 4grams solution). But since this is %, we would have to change 4 grams of solution to 100 grams. Here's one way:
1 gram NaCl x 25 = 25 g NaCl = 25% NaCl w/w
4 grams solution 25 100 g solution
Here's another way:
1 g NaCl ÷ 4 = 0.25 g. NaCl x 100 = 25 g NaCl =25% NaCl w/w
4 g solution ÷ 4 1 g solution x 100 100 g solution
Notice that when we got the denominator to 100 grams, we replaced it with "%" sign. The grams over grams is indicated with "w/w" (weight to weight or with the words, "by weight")
Notice that 150 g is not the same as 150.0 g. "150 g" is only accurate to two significant digits (15). If it was written as "150. g" then it would be 3 significant digits. Here it is "150.0 g" so that's 4 significant digits.
a) Calculate the %NaCl and %water by weight.
Remember "% by weight" means the number of grams of one compound found in 100 grams of the whole solution (or mixture). In the example above, the whole solution is made of 150.0 grams of salt plus 500.0 grams of water. So the whole solution would weigh 650.0 grams. What fraction of that whole solution is the salt? Well, that's:
150.0 g NaCl
650.0 g solution
So this is the fraction but it says 150.0 g NaCl for every 650.0 g of solution. If we want this in %, then it needs to be for every 100.0 grams of solution. We can do it the same way as the example above.
150.0 g NaCl ÷ 650.0 = 0.230769 g NaCl x 100 = 23.0769 g NaCl = 23.08% NaCl w/w (or by weight)
650.0 g solution ÷ 650.0 1 g solution x 100 100 g solution
The first step was to divide by 650 to find out the grams of NaCl per gram of solution. Then multiply by 100 to get grams of NaCl per 100 grams of solution. The "per 100" is replaced with "%" sign. The grams over grams are indicated by "w/w" or with the words, "by weight". Notice the the beginning grams had 4 significant digits, so we round the answer to 4 significant digits.
b) If you evaporated 88.840 grams of solution, what weight of salt should you get?
This could have been a trick question. If it said, "If you evaporated 88.849 grams of solution, what weight of salt is still in solution?" the answer would be 150.0 grams of salt because salt doesn't evaporate.
This question means if you take 88.840 grams of the solution described and let it dry, how much salt would be left?
We've been using the label-factor method (also called dimensional analysis), so we will use it here. We are given the fact of 88.840 grams of solution, and they want to find grams of salt. So we have to cancel "grams of solution" and introduce "grams of NaCl". Notice problem (a) above gives us the concentration of salt (fraction of salt) in our salt water solution written in four different ways. Any of those could work to cancel "grams of solution" and give us grams of salt; however, if we use 23.08% NaCl w/w, then we need to change it to 23.08 g NaCl per 100 g solution.
88.840 g solution x 150.0 g NaCl
650.0 g solution
88.840 g solution x 0.230769 g NaCl
1 g solution
88.840 g solution x 23.0769 g NaCl
100 g solution
88.840 g solution x 23.08 g NaCl
100 g solution
All of these work. (Note: The last one was rounded to 4 significant digits) All of these will cancel grams of solution (g solution) and leave us with grams of NaCl. Even though 88.840 g has 5 significant digits, the concentration is only 4 significant digits, so the answer to this problem will only have 4 significant digits.
This problem is like (b) except it's the reverse situation. Here we are given grams of salt (NaCl) and need to find the grams of the salt solution needed to give us this. We can use the same concentration of salt that we found in (a) and used in (b), except it needs to be inverted in order for us the cancel grams of salt and give us grams of solution. Let's use the last one from (b) but invert it
15.605 g NaCl x 100 g solution = ??? g solution
23.08 g NaCl
So we invert it so that the g NaCl cancels. So multiply 15.605 by 100 and divide by 23.08 to get the grams of solution needed. The above grams of solution need to be rounded to 4 significant digits because our NaCl concentration is only to 4 significant digits. There is a hitch with this problem.
2. A student measured the width of his text with a cm ruler and with his hand. The student found that it is 21.5 cm or 2.10 "hands" wide. Write the conversion factors below:
Trivia: The idea of using a hand's width as a unit of measurement is an old one. It is still the measurement of choice for height of horses.
cm/hand. For this one just put 21.5 cm on top and and 2.10 hands on bottom. Divide both by 2.10 to get 10.238 cm per hand. Round it to 3 significant digits.
hand/cm. Just reverse the cm and hands. Divide both by 21.5 to get hands per cm.
m/hand. First convert 21.5 cm to meters. Then do the same as cm/hand.
What is the width of 5.0 hands in cm and m? Use the cm/hand conversion above, followed by the m/hand conversion above.
Problems:
A. Factor-Label Problems
1. Convert 552 grams to kg, mg, (lbs).
My approach is to focus on "k" and "m" and not worry so much about kg or mg.
552 g x k = ? kg
1,000
Notice that to get kg all that was missing was the "k". "k" means 1,000, so you just set up a conversion fraction that has k on top and 1,000 on bottom. You can do the math. For "mg", we do the same approach. "m" means milli which is one thousandths (0.001)
552 g x m = ? mg or 552 g x m = ? mg
0.001 1/1000
These both come out the same. Remember if you divide by "1/1000" you have to invert and multiply (552 x 1000/1).
For grams to pounds, I always remembered by 71 Corvette that had a 454 cubic inch motor. I remember that 1 pound is the same as 454 grams (3 significant digits).
552 g x 1 lb = ? lb
454 g
Notice that the conversion factor has lb on top and g on bottom so that grams cancel and lb will end as just pounds (not per lb)
In solving these kind of problems, you look at the units of measurement and use them in a way to cancel out the units that are not in the final answer and to keep the unit(s) that are. Here are final answer is just dollars. So miles in the "420 miles" and the miles in "20.0miles/gal" must cancel. The gallons must cancel and we only need to keep dollars ($). Let's start with the 420 miles and the 20.0miles/gal. We need the miles to cancel, so we write it like this:
420 miles x 1 gal =
20.0 miles
What this does is cancel miles and gives us gallons to travel that far. It makes sense because every time 20 miles divides into 420 miles that's another gallon. Notice I wrote "1 gal" instead of "gal". Both are OK, but "20.0miles/gal" means miles per 1 gallon.
Now we can use the $0.95/gallon. This conversion factor is good because dollars is on the top and gallons in the denominator, which will cancel the gallons to travel the 420 miles: Here's what we have now:
420 miles x 1 gal x $0.95 = $
20.0 miles 1 gal
Here's the whole conversion. Miles cancel and so do gallons. Plus we end up with dollars. So we multiply all the numerators (420 x 1 x 0.95) and divide by (20.0 x 1). Remember significant digits. "20.0 miles" has 3, but 420 miles has just 2. "1 gal" and $0.95 are exact numbers because they are counted not measured. So the final answer should be rounded to 2 significant digits.
This one is pretty easy. The volume is 30.0cm x 15cm x 20cm, which is 9,000 cc. Since mL = cc, we also have 9,000 mL. (I'm assuming your instructor doesn't want you to use your conversion from earlier.) To get to liters (L), we need to cancel the "m" in "mL"
9,000 mL x 0.001 = ? L
m
Since m means milli, which is 0.001, we use that conversion factor. The "m" will cancel leaving us with just liters (L). Some instructors will show it this way:
9,000 mL x 1 L = ? L
1,000mL
This works, too because "mL" cancels leaving you with just "L". Here you know that 1 Liter is the same amount as 1,000 mL. As long as the numerator and denominator are the same size, it works.

a) What is the percent by weight of the calcium carbonate in each tablet?
In other words, what fraction of the tablet is calcium carbonate? More specifically, what fraction of the weight of the tablet is the weight of calcium carbonate (CaCO3)?
0.500 g CaCO3 ÷ 1.265 =0.395 g CaCO3 x 100= 39.5 g CaCO3 = 39.5% CaCO3 w/w
1.265 g tablet÷ 1.265 1 g tablet x 100 100 g tablet
Notice I set it up as a fraction (smaller on top). By dividing by 1.265, I get 0.395 grams calcium carbonate per 1 gram tablet. Then by multiplying top and bottom by 100, we get per 100 grams tablet. "per 100" is abbreviated as %. The grams are dropped but indicated by w/w.
b) Write the percent value as two conversion factors with units and labels.
I'm not sure here, but I think they want this:
39.5 g CaCO3 or 100 g tablet
100 g tablet 39.5 g CaCO3
With the left conversion factor, we can be given a weight of tablets and we use it to find the weight (grams) of calcium carbonate. The right conversion factor can be used to find how many grams of tablets we can make from a certain weight of calcium carbonate.
c) How many grams of calcium carbonate are in 15.2 g of TUMS.
Yes, the conversion factors above is handy for this problem. We are given the grams of the tablets, but they want grams of calcium carbonate. It looks like the left conversion factor from b) will cancel the tablet weight and give us the calcium carbonate weight.
15.2 g TUMS tablets x 39.5 g CaCO3 = ? g CaCO3
100 g tablet
Yes, the grams of tablets cancel and we are left with grams of calcium carbonate.
2. If 6.6% of a class earns A's, how many A's are earned in a class of 92 students?
Whenever you see "% of" you should think of multiplying because a fraction of something is calculated with multiplying. For example, if is said two thirds of this 600 dollars is yours. You can find that amount with 2/3 x 600. So in this problem "6.6% of a class" means 6.6% x the class. In this case it is 6.6% x 92. Or 6.6/100 x 92.
3. Rust is a compound that is 70.7% iron, and the rest is oxygen. How many grams of iron, and how many grams of oxygen are there in 680.0 grams of rust?
The question is a little sloppy here. It says rust is 70.7% iron, but it doesn't say if that means 70.7% of the weight of rust is iron, or 70.7% of the volume in rust is iron, or that 70.7% of the number of atoms in rust is iron. So that's not a clear way to express %. Reading the rest of the problem, we might assume they mean percent by weight. So that should have been written as "rust is 70.7% iron w/w ( or by weight).
So here it is 70.7% of the weight of rust (680.0 grams) is iron's weight. Mathematically, that's written
70.7% x 680.0 g rust = grams of iron
Oxygen would have to be 680.0 grams minus the weight of the iron, since oxygen has to make up the rest of the weight.