Page 88: Objectives
1. Check the pH of aqueous solutions and household products using various indicators and a pH meter.
2. Identify the buffer solution.
A. pH of solutions
pH is used to indicate the acidity of a solution and is expressed as follows:
pH = - log[H+]
where [H+] is the concentration of H+ ion (moles per liter).
There are both hydrogen ions (H+) and hydroxide ions (OH-) in water.In pure water, the amounts of each are equal and very small (1 x 10-7 M) and the pH =7.
Let's see how pH 7 comes from a concentration of 1x10-7M. Using the formula above, it says pH = - log[H+]. The [H+] is the concentration. So we have pH = -log (10-7M). To find the log of a number, you find the exponent of 10 that gives you that number. For example, the log of 1000 is asking for the exponent of 10 that gives you 1000. Well 103 equals 1000 so the exponent of the 10 is 3. So the log of 1000 is 3. In this example, a concentration of 10-7 makes it easy because it is written with 10 with the exponent of "-7". So the log of 10-7 is simply -7. The formula for pH says pH=-log(10-7), so pH=–(–7). So pH = 7.
Why not have pH simply be the concentration and not get complicated using log? The reason is that the concentration of the H+ ions in various solutions varies greatly. For example, the concentration of H+ ions in your stomach is about billion times more than the concentration of H+ ions in an anti-acid remedy like Milk of Magnesia. Instead of working with these large differences in concentration, the log of the concentration makes the values a lot smaller. In other words, pH 1 for stomach acid compared to pH 10 of Milk of Magnesia is easier to work with than using 1x10-1 molar H+ for stomach acid and 1x10-10 molar H+for Milk of Magnesia.
In an acidic solution, [H+]>[OH-], therefore the pH is less than 7. In a basic solution, [H+]<[OH-], and the pH is greater than 7. The higher the pH, the more basic the solution: the lower the pH the more acidic the solution.
There are various ways of measuring pH of a water solution. You can use litmus paper or other indicators (compounds which are a different color in acid or base) to test a solution. You can also use a pH meter which electronically measures the H+ concentration.
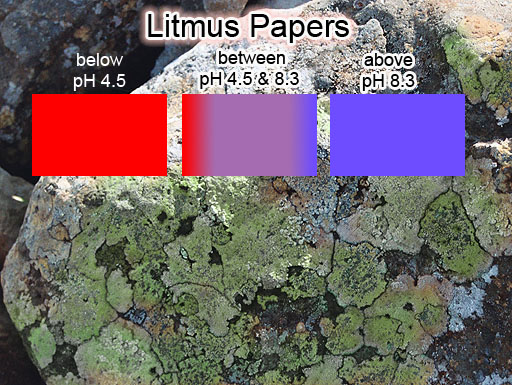
LITMUS PAPERS: Litmus paper is usually made from about 10 to 15 dyes that are extracted from lichen (a fungus). In the desert we often see rocks with lichen on the shady side of the rock. It's usually green but can also be other colors. The dyes turn blue if in alkaline solutions. However, when in acidic solutions, the dye turns to a red color. So blue litmus paper turns red if the solution is acidic (pH<4.5). Red litmus paper turns blue if in an alkaline solution (pH>8.3). In between these pH levels it is purple.



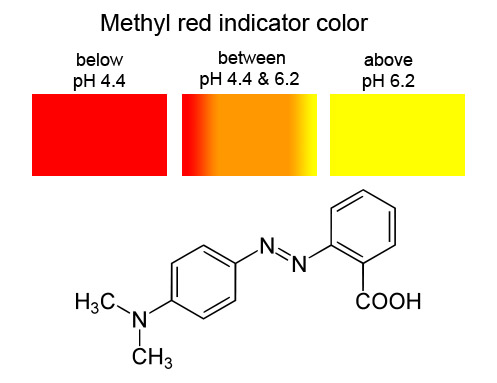
B. Buffer solutions
A buffer solution resists change in pH if a small amount of acid or base is added. Buffering actions are very important in the human body. For example, the pH value of blood must be maintained around 7.4. Buffers in blood (HCO3- and H2CO3) keep the pH value almost constant even if acidic foods are eaten or lactic acid is produced in the muscles during exercise.
p. 90. Experiment 12
A. pH measurement with indicators and pH meter
Prepare the indicator from red cabbage as follows: fill a 50 mL beaker with fresh chopped red cabbage, or 1 gram of dried red cabbage. Put the cabbage into a 250 mL beaker and add 50 mL of water. Boil gently for 10 minutes. Let the beaker cool and decant the solutions from the cabbage.

a. Measurement of pH of standard solutions and household products
While the cabbage is cooking begin your pH testing with the other indicators. Pour about 2 mL of a sample into your smallest beaker. Test the pH with litmus papers, universal paper, and the pH meter. Pour about half of this solution into a test tube and save it for testing with the cabbage indicator. To the remaining sample in the beaker, add two drops of methyl red indicator. Record the color of the solution. Discard the sample. Repeat the tests for each solution below
When your cabbage indicator is ready, add a dropper full of cabbage indicator to each solution test tube. Again record the colors.
Solution |
Indicators |
|||||
pH Meter |
Universal pH paper |
Litmus paper |
Methyl red* |
Cabbage* |
||
Red |
Blue |
|||||
A |
||||||
B |
||||||
C |
||||||
D |
||||||
Lemon juice |
red? |
red? |
red? |
reddish? |
||
M.O.M |
blue? |
blue? |
yellow? |
greenish? |
||
Tap water |
red/purple? |
blue/purple? |
orange? |
purplish? |
||
Milk |
red/purple? |
blue/purple? |
orange? |
purplish? |
||
* Do not use methyl red and cabbage indicator with the same sample (same container).
Lemon juice: Lemon juice is reported to be between pH 2 and 3. Litmus paper is suppose to turn red in acid solutions. Methyl red is suppose to be red below pH 4.4. Cabbage indicator ought to be reddish. If the lemon juice is heavily diluted, then these colors might not be accurate
M.O.M. (Milk Of Magnesia=magnesium hydroxide solution) is reported to have a pH between 10 and 11. Blue litmus paper should remain blue but red litmus paper should turn blue. Methyl red is yellow above pH 6.6. Cabbage indicator is probably greenish.
Tap water is supposed to be neutral. The universal pH paper should indicate pH 7. The red litmus paper ought to turn more purple (or not change). the blue litmus paper ought to become purple (or no change). Methyl red is probably orange. Cabbage indicator should be purplish.
Milk is reported to have a pH between 6 and 7. The color of the pH indicators should be the same or close to that of tap water.
B. Buffer solution
Prepare a baking soda solution by dissolving about a teaspoon full of baking soda (NaHCO3) into 50 mL of distilled water. Take the pH of the solution and record. Divide the solution into two portions. To one portion, add 2 drops of 1M HCl and swirl to mix. To the other, add 2 drops of 1M NaOH and mix. Take the pH of each solution with the meter and record.
Prepare two beakers with 25mL of distilled water in each. To one beaker, add 2 drops of 1M HCl and swirl to mix. To the other, add 2 drops of 1M NaOH and mix. Take the pH of each solution with the meter and record.
pH Value |
||
| System tested | Baking soda solution |
Distilled water |
| Original | Predicted to be between 11 and 12 but record pH meter value |
7.0 |
| After HCl addition | Predicted to stay close to original value but record pH meter value |
Predicted to be between 2 and 3, but use pH meter value |
| After NaOH addition | Predicted to stay close to original value but record pH meter value |
Predicted to be between 11 and 12, but use pH meter value |
| Using your results, discuss which solution showed better buffering action. The baking soda solution does a better job because the HCO3- in solution will absorb added H+ to form H2CO3. The HCO3- in solution will donate H+ to neutralize the OH- from NaOH. In both cases, they resist a change in pH. Water showed no buffering ability. |
||
There is a way to estimate the pH of the water that had 2 drops of acid added. It usually takes about 30 drops to make 1mL, so the 25mL of water is equal to about 750 drops (25mL x 30drops per mL). The 2 drops of 1M HCl is now diluted to 752 drops. So 2 drops compared to 752 drops is like 1 compared to 376. So it is now 376 times weaker. So 1M divided by 376 is 0.00266M. The pH = -log(0.00266). Using a calculator the log of 0.00266 is -2.58. So pH=-(-2.58) or pH=2.58. The accuracy of 2 drops isn't very good, so the estimate pH is between pH 2 and pH 3. For the NaOH, the same thing can be done. The final concentration of NaOH is 0.00266M. To get the acid concentration, we use the fact that the multiplication of the OH- concentration times the H+ concentration is always 10-14. So [0.00266 OH-] x [H+] = 10-14. To solve for H+ concentration we divide both sides by 0.00266. We get 3.76x10-12 as the molar concentration of H+ (the acid). To find pH, we use the formula of pH=-log(3.76x10-12). That solves as pH 11.42. So the estimate is between 11 and 12 for the water that has had 2 drops of 1M NaOH added.
The baking soda should show better buffering action because some of the baking soda will neutralize the acid or base. First let's see what happens when baking soda is added to water. Baking soda is NaHCO3. In water it forms Na+ and HCO3-. Water (H2O) is often written as HOH because it has a tendency to be pulled apart to form H+ and OH-. Since opposite charges attract, Na+ will pull on the OH- to form NaOH, and HCO3- will pull on H+ to form H2CO3. NaOH quickly gets pulled apart by water to turn back into Na+ and OH-. In contrast, the H2CO3 (carbonic acid) is stable in water and some of it resists the pull of water and remains intact. What this means is that the H+ is removed from the solution as H2CO3, but the OH- is still floating around. For this reason, a solution of NaHCO3 will be basic (alkaline) because more OH- than H+ is in the solution. If acid (H+) is added to the a solution of baking soda, the extra OH- in the solution will immediate neutralize the H+ by forming H2O (OH- + H+ -> H2O). Also, the HCO3- in solution can grab the H+ to form H2CO3. This is the buffering action of the baking soda solution on the addition of acids.
The above paragraph discussed the buffering of added acid. To buffer added base (added OH-), the HCO3- in the baking soda solution will split into H+ and CO32- to let the H+ combine with the OH- to make water.
C. Carbon dioxide
In your cells, the oxygen you breathe is used to change glucose, C6H12O6, into carbon dioxide and water through a series of reactions catalyzed by enzymes.
Check the carbon dioxide in your breath.
1. Place a very small volume (about one eight of a dropperful) of calcium hydroxide solution into a test tube and check its pH by adding 2 drops of phenolphthalein indicator (colorless in acid, magenta in base). Record the color. (A saturated solution of calcium hydroxide is often called "lime water". No, that has nothing to do with lime flavor).
2. Use a straw to blow bubbles through the solution until the color changes. Record your observation.
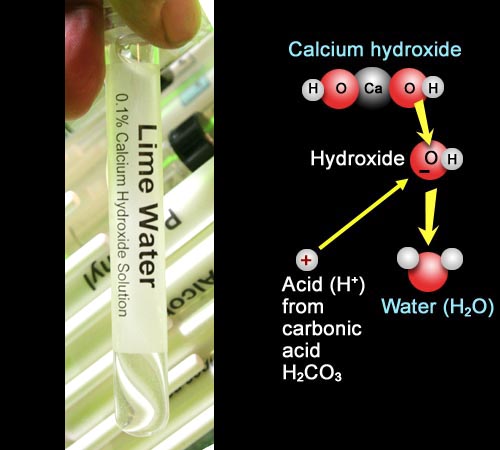
Calcium hydroxide dissolves to make Ca2+ and OH-. H+ from the carbonic acid formed by CO2 in water will react with the OH- to form water. In this way the CO2 from the breath will neutralize the hydroxides.
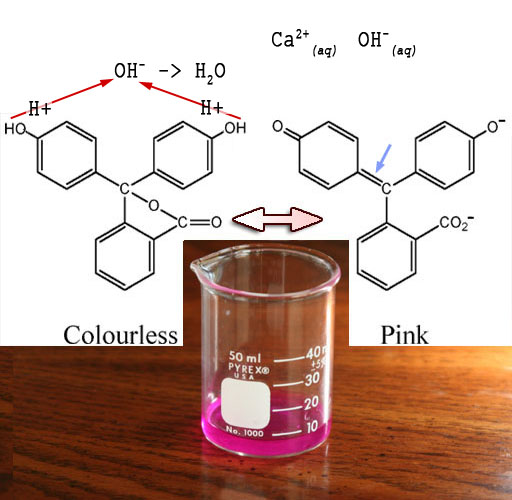
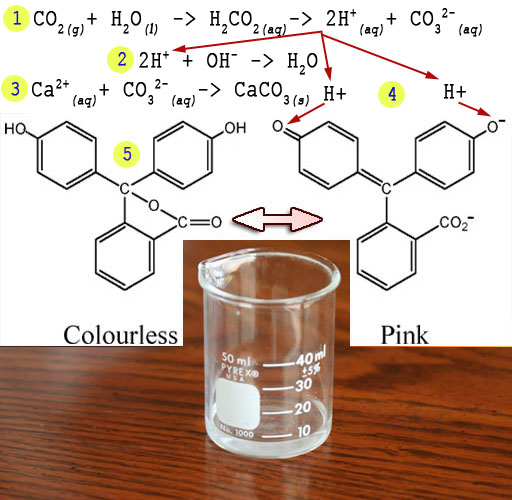
Here's the steps of how your breath turns the purplish solution to clear.
1) CO2 combines with water to make carbonic acid which ultimately breaks down into acid (H+) and carbonate (CO32-).
CO2(g) + H2O(l)> H2CO3 > CO32-(aq)+2H+(aq)
2) The acid neutralizes the OH- from the lime water.
2H+(aq)+ OH-(aq)> H2O(l)
3) The carbonate and calcium combine to make chalk
CO32-(aq)+ Ca2+(aq)> CaCO3(s)
4) Additional H+ created attaches to the phenolphthalein returning it to its clear form (5).
Note: (g)=gas, (l)=liquid, (aq)=aqueous, i.e, dissolved in water, (s)=solid.
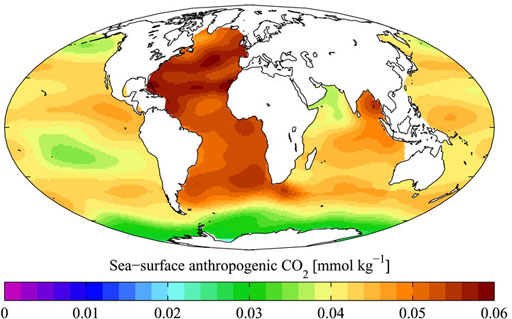
As CO2 increases in the atmosphere due to combustion of fossil fuels, what will happen to the pH of water in rain, ponds, and oceans?
The short answer is that the pH of the water will go down (become more acidic) due to dissolved CO2 that converts to carbonic acid.
On the left is a graph of the current CO2 levels in the ocean caused by human activity (anthropogenic). Notice the Atlantic Ocean has the highest levels probably because of the heavily populated cities on that coastline plus the high amount of intercontinental ship traffic.
Page 91:
Questions
1. Given the following pH values: 9, 4.5, 2, 11, 3.8.
a) Arrange the pH values in terms of increasing acid strength. (Remember the lowest acid strength is the highest numbers. So "11" would be the least acid strength. You can turn the pH values to concentration easily. The concentrations are 10-9, 10-4.5, 10-2, etc. Note that 10-2 is 1/100 [hundredth], which is a larger number than 10-9, which is 1/1000000000 [a billionth])
b) Arrange the pH values in terms of increasing base strength. (Base strength is reverse of acid strength, so the order in 1a is reversed)
2. Define the following terms:
a) buffer
In chemistry it means a substance added to a solution to resist the change in the pH of the solution.
b) exothermic
A chemical change that releases heat.
c) endothermic
A chemical change that absorbs heat.
2. Explain why you take M.O.M. (milk of magnesia) when you have a stomachache.
Many stomachaches are acid indigestion caused by excess acid. Milk of Magnesia is a solution of magnesium hydroxide. The hydroxide ions can neutralize excess acid in the stomach.
3. If you test a solution of pH=8.5 with the following indicators, what colors do you expect to observe?
Using the charts above, we can predict the colors.
Indicator |
Color |
methyl red |
yellow |
blue litmus paper |
blue |
cabbage solution |
blue |
5. On the pH scale below, write the names of the household products tested above, with their appropriate pH values (write sideways). (transfer your data from the table to the chart in the lab manual)
6. Criticize the statement "We must reduce the pH of our rivers to zero."
The pH scale is not like typical concentrations measurement such as parts per million, moles per liter, or grams per 100mL. In those cases zero means zero level. The person making this statement believes that a zero pH means zero acid. But pH 0 means 10-0 moles per liter. 10-0 equals to 1, so it means 1M or 1 mole of H+ ions per liter. 1 mole per liter is a pretty strong acidic solution (10 to 100 times stronger than stomach acid). Another problem with getting acid concentration in water to zero is that it is impossible. To approach zero acid levels, the water would have to have extremely high levels of hydroxide ions. In other words, a concentrated solution of lye (NaOH) would push the acid (H+) levels in water close to zero. There would be no danger from acid but the alkaline levels would be so high as to dissolve skin and hair.