CHM130 Lab Final for online CHM130LL
The lab final is now moved to Sapling Learning so that the grading can be immediate. Do not do the below questions. Those are now in Sapling Learning.

Chemistry and Survival Skills:
Survival in the wilderness depends on many things, and a knowledge of science and chemistry is definitely an asset when trying to survive. An emergency or survival kit is also helpful. Let's say you don't have an emergency or survival kit, but you do have the portable chemistry kit that you got for the online CHM130 lab.
The exam tests your ability to use the items in the kit combined with your lab skills and knowledge of chemistry to solve some survival issues.
Getting lost in the desert in the summer or having your car get stuck on some remote road in the winter are two times that survival skills are very important.
If your element code name starts with A-G, then email your answers to Loree Cantrell-Briggs at lor2060912@phoenixcollege.edu. If your element code name starts with H-Z, then email your answers to Quinn Thacker at QRT2004@yahoo.com.

In a cold environment, getting shelter and keeping warm has a priority. Wrapping up in extra clothes or blankets and getting out of the wind are the first things to do. Then you start looking for a source of heat. That's when the kit will help.

The kit has an alcohol burner with ethanol fuel plus candles, butane lighter, mineral oil, and biodiesel that can also burn. Let's do an assessment of the calories of energy available from these sources.

Heat of combustion from ethanol is 7,000 calories per gram. Your kit has 125 mL of alcohol burner fuel which is mostly ethanol. The density of ethanol is 0.8 grams per mL. So multiplying 125 mL x 0.8 grams per mL cancels the mL and gives us 100 grams of ethanol.
1) How many calories of energy is in that 100 grams of ethanol?

Your kit has 3 small candles. Use your digital balance to find their total weight (Ignore mass of the aluminum holders). Heat of combustion from wax is 9,000 calories per gram.
2) How many calories of energy is in the wax of your 3 candles?

Your kit has a butane lighter. The heat of combustion of butane is 12,000 calories per gram. The lighter holds 8 mL of butane. Density of liquid butane is 0.6 g/mL. Use density to find grams and then multiply by 12,000 calories per gram to find calories.
3) How many calories would be gotten by burning the 8 mL of butane?

Your kit also has 40 mL of biodiesel. It can be burned by making a wick from a strip of cotton, placing the bottom half of the cotton wick into the biodiesel and then lighting the wick. The combustion of biodiesel produces energy similar to oils, fats, and waxes. So it is also 9,000 calories per gram like wax. In your kit, some glycerin sits at the bottom of the biodiesel. Glycerin will burn, but its combustion products are not healthy. So just burn the biodiesel. The density of biodiesel is 0.9 grams per mL. Use density to convert to grams, then find the calories.
4) How many calories could be released by burning the 40 mL of biodiesel (Round to 1 significant figure)?
All of the above sources are good for heat; however, if there is firewood around, using them to start the fire could be a better use of their flammability. However, wood fires put out a lot of carbon monoxide. The burning of above compounds has much less carbon monoxide, so those could be used inside of car with less chance of carbon monoxide poisoning. The below reaction is for the complete combustion of ethanol. Balance the reaction.
CH3CH2OH(l) + O2(g) → CO2(g) + H2O(g)
5) Write the balanced equation for the combustion of ethanol.

If the butane lighter doesn't work, having these above sources of heat will not be helpful if you can't light them. Fortunately, the kit has a Fresnel magnifier, which acts like a magnifying glass. This can concentrate the sun's light and start a fire. It works best if the paper or item is dark in color. If a newspaper, focus on any dark area. The focal length of a lens is the distance from the lens that the light source (such as sun or light bulb) is focused. Go into a room that has a light bulb on. Let that light pass through the Fresnel lens and fall upon a sheet of white paper. You will see the image of the light on the white paper. The distance the paper is from the lens when that image is most focused is the focal length. Measure that length to the nearest centimeter.
6) What is the focal length of the Fresnel lens in your kit to the nearest centimeter?


Starting a fire using a magnifying glass is best accomplished using "char" cloth because char cloth is black, and because it can ignite very easily. Even a small spark can ignite it. Char cloth is a piece of cotton cloth that has been heated without exposure to oxygen. This causes a pyrolysis of the cotton fibers which releases water leaving mostly carbon behind.
7) Look up how char cloth is made and report how you would create char cloth using the items in your kit. The cloth would come from some clothing you are wearing, but the other items would be in the kit.

In the lab that covered Heat of Solution, you discovered that lithium chloride produces quite a bit of heat when it dissolves in water. So it could be a good hand warmer. The literature lists the Heat of Solution of LiCl as -37 kilojoules per mole. The negative sign indicates that dissolving releases energy rather than absorbing it. Your kit has about 7 grams of lithium chloride, but lets say we weighed it as 7.35 grams.
8) How many calories of heat could we get from dissolving it (report in proper number of significant figures)?
The below spreadsheet will help you with the calculations. You need to find the molar mass of LiCl and fill in the missing data in cell I2. Then do the math.
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
|
| 1 | mass LiCl |
grams to moles |
Heat of solution |
joule>calorie |
cancel k |
calories from dissolving |
||||||
| 2 | 7.35 |
grams | 1 |
mole LiCl | 37 |
kilojoules | 1 |
calorie | ? |
= |
?? |
calories |
| 3 | ?? |
g LiCl | 1 |
mole LiCl | 4.184 |
joules | kilo |
|||||
9) What did you put into cell C3?
10) What did you put into cell I2?
11) What units canceled in the dimensional analysis above?
A joule is the energy released using one watt of electrical power for one second. Since a mole of lithium chloride produces 37.03 kilojoules of energy when dissolving, that means it produces the equivalent of 37 kilowatts of power for one second, or 37 watts for 1000 seconds, or some combination that multiplies out to 37,000.
12) After the lithium chloride dissolves and you get the heat from the Heat of Solution, what could you do to the dissolved lithium chloride so that you could reuse it for heating? (Hint: The answer is pretty simple).

Summer in the desert can be brutal. Finding shade is the first thing to do. Unfortunately, a car may provide shade, but the windows and metal make it more like a solar oven. Under a tree or behind a rock are better shade structures.
The body sweats so that the evaporation of water (sweat) will absorb heat from the skin. Water absorbs 540 calories of heat for every gram that evaporates (Heat of Vaporization). Your kit has 125 mL of purified water. Since water is basically 1 gram per mL, the kit has 125 grams of water. If you didn't need to drink the water, you could use it to stay cool.
13) How many calories of heat would be absorbed by 125 grams of water evaporating?
If the day is humid, the evaporation of water is slow and the cooling is not very effective. The alcohol burner's fuel is mostly ethanol. That evaporates much more readily giving a much better cooling affect. Placing some on the back of the neck is effective. The kit has 100 grams of the alcohol, which absorbs 200 calories of heat energy per gram as it evaporates.
14) How many calories of heat could be absorbed as the 100 grams of ethanol evaporates from the skin?

People can only last about 3 days without water. So water is another priority. The kit has 125 mL of purified water, so that's some water, but you would have to find more. Also, the water needs to be free of bacteria, other microbes, and any toxic metals. The kit has items that can help with disinfecting water.
Related to disinfecting water is disinfecting any wounds or cuts.

The standard way of disinfecting water is to boil it; actually just getting it to the boiling point is good enough. The approximate energy to raise water temperature is 1.0 calories for each gram and for each degree Celsius (That's called the Specific Heat of Water). If the water you find is 20.°C, then it needs to go up 80°C to reach 100°C. The large beaker holds 250 mL of water, which we can assume is 250 grams of water.
15) How many calories will it take to get the 250 grams of water to go from 20°C to 100°C (round to 2 significant figures)? (Remember, one calorie for each gram and one calorie for each degree Celsius).
16) If you used the alcohol burner to heat the water and all of the heat from the burning of the alcohol went into heating the water, how many grams of alcohol will you burn to do that heating? (Remember, for every 7,000 calories needed, it will use up a gram of alcohol.)

In winter survival, they say not to eat snow when thirsty because the melting of the snow in the mouth will absorb too many calories of heat from the body. You need to melt the snow using an external heat source.
If you pack 250 mL beaker full of snow, it will melt down to about 1/5 of that volume. In other words, it will end up as 50 mL of water, which we can call 50 grams of water. The Heat of Fusion of water (heat from going from ice to liquid or liquid to ice) is 80 calories per gram of water (snow in this case).
17) How many calories will be needed to melt that 50 grams of snow (report as 1 significant figure)?
Of course the melted snow will be very cold. So we need to warm it from 0°C to a very warm 50°C so that drinking the water will provide warmth and hydration.
18) How many calories are needed to warm the 50 grams of water from 0°C to 50°C? (refer back to problem 10)
19) Would you call Specific Heat a physical property or a chemical property?

Silver ions are used in many places to combat bacteria. One company called Agion specializes in using silver ions to kill bacteria. The name "Agion" is based on "Ag ion" meaning the silver ion. The company coats cloth, plastics, and other materials with a binder (often a clay) that contains silver ions. You may have seen ice makers at soda fountains with the Agion emblem on them.
Your kit has a 0.1 M silver nitrate solution. (0.1 M is 0.1 mole/liter). Apparently, a couple of drops of this solution per liter of water will provide some disinfection of the water, and the level of silver will be low enough to drink (at least in an emergency situation).
20) If 2 drops is 0.07 mL, how many grams of silver ions is in the two drops of 0.1 M Silver Nitrate?
The below dimensional analysis setup will help you solve the problem, but you need to look up the molar mass of silver. Note: the silver ion is the same mass as silver.
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
|
1 |
0.1 M AgNO3 |
1 Ag in AgNO3 |
converts moles Ag to grams |
2 drops |
cancel m |
g Ag+ in 2 drops 0.1M AgNO3 |
||||||
2 |
0.1 |
moles AgNO3 | 1 |
mole Ag+ | ??? |
gram Ag+ | 0.07 |
mL | 0.001 |
= |
?? |
grams Ag+ |
3 |
1 |
Liter | 1 |
mole AgNO3 | 1 |
mole Ag+ | milli |
|||||
21) Cell B3 has "Liter". What cell cancels out the liters?
22) Cell D2 has "moles Ag+. What cell cancels moles of Ag+?
You may have used Tincture of Iodine as an antiseptic for a cut. Tincture of Iodine is a mixture of elemental iodine (I2) and potassium iodide (KI) in a solution of water and ethanol. Your kit has potassium iodide but no elemental iodine; however, you can convert the iodide ion (I-) into elemental iodine (I2) using the 9V battery that powers one of the LED light strips in the kit. Plus, you have some wires with alligator clips. A pencil is not in the kit, but you may have one of those with you. Or you could use the stainless steel microspatula instead of a pencil.
The positive end of the battery pulls the electron off of two iodide ions (2I-) which leaves elemental iodine (I2). The negative end of the battery gives an electron to water, which causes it to split into hydrogen gas (H2) and a hydroxide ion (OH-). Below are the half reactions and the net reaction
+ side of battery: 2I-(aq) → I2(s) + 2e-
- side of battery: 2H2O(l) + 2e- → 2OH-(aq) + H2(g)
net reaction: 2I-(aq) + 2H2O(l) → I2(s) + 2OH-(aq) + H2(g)
23) The salt in the water was potassium iodide (KI). Why wasn't the potassium ion (K+) shown in these equations?
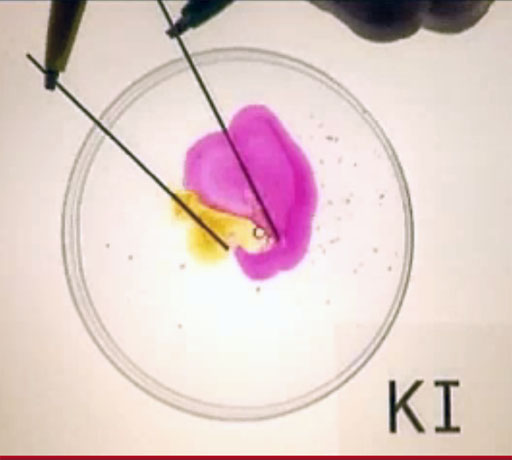
Use the below link to go to Youtube video to see this happening:
http://www.youtube.com/watch?v=LGwPfo_eunY
24) Which side (purple or yellow) had the hydrogen gas bubbling from it?
Your kit has the phenolphthalein that was used to show the OH- ions were being formed. Phenolphthalein turns purple in an alkaline solution (excess OH- ions). It's not needed for the purpose of making iodine, but it was useful in the video to see the production of OH-.
Iodine (I2) has low solubility in water, so by adding iodide (I-), from KI, a reaction that forms the triiodide ion helps bring the iodine (I2) into solution (see below). Also, instead of using just water, a 50% v/v of ethanol and water helps dissolve iodine. Your alcohol fuel can supply the ethanol. This Tincture of Iodine solution is a good antiseptic for cuts and wounds, and a few drops added to a liter of water will help disinfect the water.
I2(s) + I-(aq) ![]() I3-(aq)
I3-(aq)
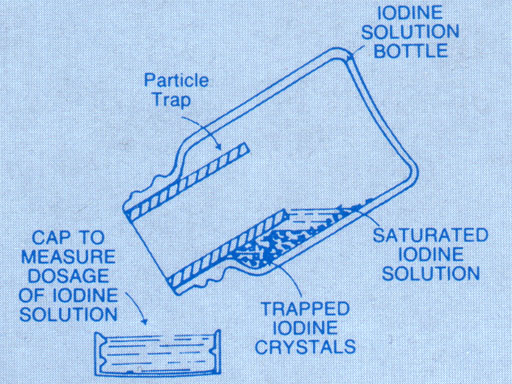


In the online CHM107 lab, iodine is created from exposing potassium iodide soaked strips to ozone in the presence of some moisture (H2O). Here is the reaction:
KI(s) + H2O(g) + O3(g) → KOH(s) + O2(g) + I2(s)
26) Balance the above reaction.

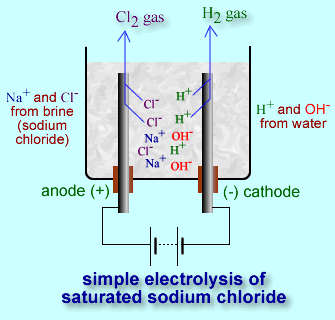
Sodium hypochlorite (NaClO), which is bleach, is used to whiten clothes but also used as a disinfectant for water. It can be made in a similar way that iodine was made using the 9 volt battery. Instead of KI salt, sodium chloride (NaCl) salt is used. Chlorine gas forms at the positive side of the battery. Like before, the negative side of the battery produces hydroxide ions and hydrogen gas. So both sides will show bubbles.
+ side of battery: 2Cl-(aq) → Cl2(g) + 2e-
- side of battery: 2H2O(l) + 2e- → 2OH-(aq) + H2(g)
net reaction: 2Cl-(aq) + 2H2O(l) → Cl2(g) + 2OH-(aq) + H2(g)
Sodium ions (Na+) are present, but they aren't reacting with anything. Also, we still don't have the sodium hypochlorite we wanted. However, if the two electrodes are brought close to each other, the chlorine gas will react with the hydroxide ions to form hypochlorite ions (ClO-). Below is that reaction.
Cl2(g) + 2OH-(aq) → Cl-(aq) + ClO-(aq) + H2O(l)
The original sodium ions from NaCl, are still present.
So the final mixture is actually, NaCl(aq), NaClO(aq), and H2O(l) so we finally get sodium hypochlorite, which will disinfect water.
27) The negative side of the battery will cause phenolphthalein to turn purple; however, when the last reaction occurs where chlorine gas reacts with the hydroxide ions, what will the phenolphthalein do then?
28) If the solution gets hot (over 50°C), sodium hypochlorite is converted to sodium chlorate, which has less disinfection properties. What is the formula for sodium chlorate?

Another way microbes can be killed is with ultraviolet light. The sun provides plenty of UV light. So placing water in the sun for about 6 hours will kill most microbes. The below article talks about how they use plastic PET bottles to hold water while being exposed to the sun. Your kit doesn't have any PET bottles, but it has the glass beakers that let UV light pass through.
29) What does PET stand for and what code number do items show that are made from PET? (PET is also abbreviated as PETE).
30) The article lists 3 principles for how UV radiation kills pathogens. What are they?

Your kit has UV detection beads. If you had another container that wasn't made of PET, you could place the UV beads in that container to see if they change colors. If they do, it means the UV light does pass through the walls of the bottle and that container would be good for sun sterilization. (Note: Your UV beads are in a test tube.)
Find the UV beads in your kit to do the next test.

Your kit has a UV light source in the mini microscope. At the bottom of the small microscope is a small light switch. Sliding it one way turns on a small ultraviolet LED light. Moving the switch the other way turns on two bright LED bluish white lights. The small UV LED light may not be strong enough to provide disinfection except for a small area.
Run the UV light on the microscope up and down half of the test tube that's filled with the UV detection beads. Do that for about half a minute. If the beads in that half of the test tube start to turn colors, then the UV light on the microscope does have some UV sterilization capability and could be used to sterilize a cut or a utensil.
31)
Report what you see regarding the changing of any colors on the UV beads.

All of the above treatments of water kill pathogens in the water; however, none of them remove any toxic metals from the water. A quick way to see the concentration of metal salts in the water is to use the TDS meter. The TDS meter doesn't say if the salts are toxic or not, but it does indicate the concentration of dissolved solids such as salts. If the reading is low (around 50ppm), then the water has the same level of salt as bottles of drinking water. If the reading is over 500ppm, then the chances of drinking some undesirable metal ions is over 10 times higher.
32) In the mud hole shown, does the TDS meter measurement include the sediment that is suspended in the water, or does it just test the dissolved solids in the water?

chloride |
bromide |
iodide |
sulfide |
hydroxide |
carbonate |
nitrate |
sulfite |
sulfate |
phosphate |
acetate |
|
Cl- |
Br- |
I- |
S2- |
OH- |
CO32- |
NO3- |
SO32- |
SO42- |
PO43- |
C2H3O2- |
|
| H+ | aq |
aq |
aq |
solid |
liquid1* |
gas2* |
aq |
gas3* |
aq |
aq |
liquid |
| Na+ | aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
| K+ | aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
| NH4+ | aq |
aq |
aq |
aq |
gas4* |
aq |
aq |
aq |
aq |
aq |
aq |
| Ag+ | solid |
solid |
solid |
solid |
solid |
solid |
aq |
solid |
solid |
solid |
solid |
| Mg2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Ca2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
solid |
solid |
aq |
| Ba2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
solid |
solid |
aq |
| Fe2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Fe3+ | aq |
aq |
solid |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
solid |
| Co2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Ni2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Cu2+ | aq |
aq |
solid |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Zn2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Hg2+ | aq |
solid |
solid |
solid |
solid |
solid |
aq |
solid |
solid |
solid |
aq |
| Cd2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Sn2+ | aq |
aq |
solid |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Pb2+ | solid |
solid |
solid |
solid |
solid |
solid |
aq |
solid |
solid |
solid |
aq |
| Mn2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Al3+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
33) Toxic Hg2+ in contact with carbonate ions forms insoluble mercury(II) carbonate. Write the double-replacement reaction of HgCl2 reacting with Na2CO3.
34) Adding sodium carbonate to the water will also precipitate out some of the lead ions in the water. Write the double-replacement reaction of PbCl2 reacting with Na2CO3.
35) Barium sulfate is extremely insoluble, so adding sulfate ions will get the toxic barium ions out of the water. Your kit has copper(II) sulfate and iron(II) sulfate, but that would add undesirable copper and iron ions to your water. What other compound in the kit would supply sulfate ions so that barium ions will precipitate out as barium sulfate?
Another way to get rid of lead ions is to use a single replacement reaction. There are 2 metals in the kit that can do that. When they react with the Pb2+ ions they will dissolve and the Pb2+ ions will form solid metallic lead, which will either plate out on those metals or form particles that sink to the bottom of the container. One of the metals in the kit is zinc (coated on the galvanized nails).
36) What is the other metal in the kit that will give its electrons to Pb2+ ions so that the lead ions will come out of solution?
37) Your kit has some heavy gauge copper wire. Will copper give its electrons to mercury ions so that mercury ions will be removed from the solution? If so, then write the single replacement reaction for copper metal reacting with mercury(II) chloride?
38) Your kit has lithium chloride and sodium chloride. If mixed, does the lithium ion give its electron to the sodium (Na+) ions to produce sodium metal?

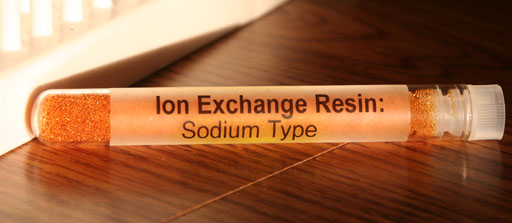
Your kit has two other items that can trap any toxic metal ions in the water. One is the ion exchange resin: dual type; which removes positive metal ions and replaces them with H+ ions. That resin also removes negative ions like Cl- and replace them with OH- ions. The other ion exchange resin in the kit is the sodium type. It removes positive metal ions and replaces them with Na+ ions. The only problem is that there is only enough resin to clean up a cup or two of water.
Read the first part of the below lab from CHM107 down through the part where it describes ion exchange:
http://www.chemistryland.com/CHM107Lab/Lab5/Filters/Lab5Exp2filters.html
Remember the words, "Ion Exchange" means that it has to exchange the contamination it takes out of the water with something it must put back into water. For example, if it pulls out lead ions, it has to put back some other positive ion such as H+ ions or Na+ ions.
39) If the ion exchange resin: sodium type removes a million Pb2+ ions from the water, how many Na+ ions does it put into the water?
40) If the ion exchange resin: sodium type removes a million K+ ions from the water, how many Na+ ions does it put into the water?

The pH of the water you find is also important. Many salts are more soluble in acidic water, which would mean that your water is more likely to contain dissolved metal salts. Your kit has 0-14 pH paper.
41) If the water was checked and found to have a pH of 6, is that acidic or alkaline?
42) If your water had a pH of 9 and you wanted to bring it to a pH of 7, what in your kit could you use to do that?
43) If you added a few drops of Phenolphthalein from the kit and the water turned pinkish, is the water acidic or alkaline?

When you are trapped in the wilderness and a search party comes looking for you, getting noticed is what you want.
In some survival kits, they have potassium permanganate. One of its uses is to make water a deep purple color. The purple water is poured onto the snow to spell out HELP. Your kit doesn't have potassium permanganate, but it does have some pH indicators which cause water to have a color at different pH levels.
Your kit has 0.1 M HCl, so two of the indicators you have will turn yellow in water that has been made acidic using HCl. Your kit also has phenolphthalein which turns water pink to purple when the pH is above 8.2. Your kit doesn't have any metal hydroxides to make an alkaline solution, but it does have sodium carbonate. Notice that when dissolved, some water molecules will split to give an H+ ion to CO32- to make the bicarbonate ion (HCO3-) and another ion, which makes the solution alkaline; and that makes the solution purple.
2Na+(aq) + CO32-(aq) + H2O(l) ![]() 2Na+(aq) + HCO3-(aq) + ???(aq)
2Na+(aq) + HCO3-(aq) + ???(aq)
44) What is the "???" ion in this equation?
The purple phenolphthalein water can be poured onto the snow to spell out HELP and make it easy for rescuers to see.
45) If your kit had potassium permanganate, it could be added to the glycerin in the kit. What happens when those two compounds are mixed?
Your kit also has 2 strips of magnesium ribbon. Magnesium is the main ingredient in flares because it burns with an intense white flame. So it's a great item to get attention especially when there is a night rescue.
46) If you coat the magnesium ribbon with lithium chloride, you will get an extra color besides white in the flame. What color is that?
47) If you coat the magnesium ribbon with copper(II) sulfate, what extra color or colors might you see besides white in the flame? (Hint: See Lab 6)


The other way to get noticed is to start a fire and create some smoke. The ethanol in the alcohol burner burns so cleanly that its hard to see. One reason for that is ethanol vaporizes easily, which allows oxygen molecules to surround it and burn more efficiently. Ethanol also has an oxygen atom in the molecule which contributes to it burning cleanly. So you need to burn substances that don't evaporate, have long carbon chains, and have little or no oxygen atoms in the molecule. Your kit has mineral oil, which is a hydrocarbon having 15 to 40 carbons and no oxygen atoms, so that's a good candidate.
48) What is a hydrocarbon?
The Native Americans placed damp grass on a fire to make smoke signals. The dampness of the grass released water which interfered with oxygen getting to the burning grass. That caused incomplete combustion, which produced a lot of black carbon soot rather than invisible CO2. They also placed a blanket over the fire which blocked oxygen which created more smoke to be released when the blanket was removed.
The best way to create smoke is to first have a hot fire using dry wood or some other flammable material (like the alcohol fuel). Then items like oil, wet leaves, shavings of a tire, or plastic are added. Smoke will form as these heat up to produce soot, other unburnt particles, and even droplets of tar and other liquids that don't burn easily.
49) What is tar?
Mineral oil and glycerin are often used in fog machines at concerts to produce a smoke affect. Both of those are in your kit. These liquids are not burned, but simply sprayed onto a hot piece of metal so that they vaporize and then condense to form droplets in the air.
50) Would you call this way of making fog a physical change or a chemical change?
Good news, the rescue party saw the smoke and found you. It seems all of your skills in survival paid off.
Well it seems that you survived your ordeal. Not being lost in the wilderness ordeal, but the lab test ordeal. On your next camping trip or road trip, we hope this test made you more aware of how to be prepared in case of being lost or broken down in some remote area.