Lab #1: Preparing solutions for use in future labs.
Experiment #2: Preparing Red Cabbage Extract

Let's begin with the items that you will need for the lab that you may need to buy at the grocery store. There are three items.
#1. Distilled water: This is not the same as bottled drinking water. Distilled water is made from steam that is cooled and collected. It is supposed to be free of any salts (minerals). An alternative to distilled water is deionized water. Deionized water is water that has been passed through resins that capture all dissolved salts. You use these to rinse off glassware so there are no mineral spots. It is also used where you want pure water.


#3: Isopropyl alcohol. This is used as a preservative for the cabbage extract and for washing out used test tubes. If you rinse them out with rubbing alcohol, it makes them dry faster. Isopropyl alcohol at 70% concentration is called rubbing alcohol. If you find isopropyl alcohol at a higher percent, like 90%, get that.

#4: Baking Soda: Baking soda when mixed in water will make it alkaline (also called basic), which means it is the opposite of acid. (explained more farther down). It will also be used to test the red cabbage extract.


#6. A few empty test tubes: You will use these to collect purified water and the red cabbage extract.

#7. Two plastic dropper bottles. One labeled pH Indicator (cabbage extract) and the other label Purified Water. In this experiment, we will be extracting the red pigment from the cabbage and placing it in the pH indicator bottle. Both bottles have a nozzle in them. Just like the Purified water bottle, the pH indicator bottle may need a spatula or fingernail to get the nozzle out of the bottle.
#8. Funnel. Your kit has a funnel and you might still have the small plastic tube still connected to it from the last experiment. You can pull off the plastic connector because it will be easier for the funnel to fit into the test tube without it.






If it looks like there's not enough water to cover the leaves, or too much of the water is boiling way, add a little more of the distilled water. (Perhaps another 50 mL)
Keep it at a gentle boil for about 10 minutes, or until the water turns a purple color. Then turn off the heat and let it cool down.
(Take a picture at this point. See if you can set your camera to either 1600x1200, 1280x960, or 1024x768. If you own a 2 megapixel camera, just use the highest setting [3 stars].)

While the cabbage is cooling, get a piece of filter paper out of the jar in your kit.
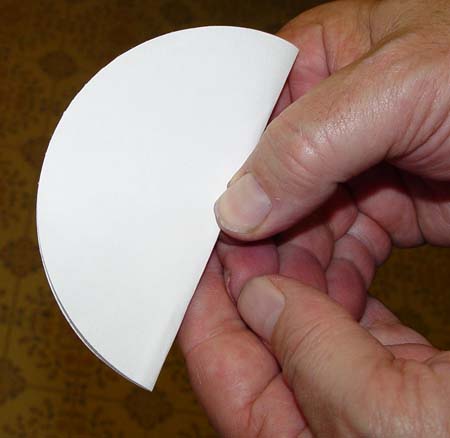
Fold the paper in half.
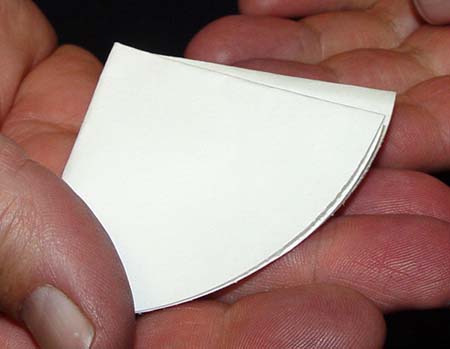

Open the filter paper so just one half side is opened.
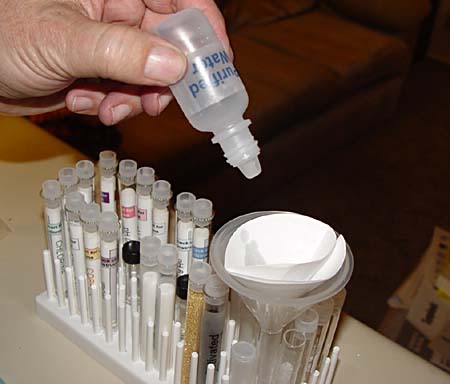


Pour the cabbage extract (juice) into the funnel with the filter paper. The liquid will go through fairly fast. When the liquid starts to fill up the test tube, move the funnel over to an empty test tube right next to it.
Again, because something might spill, I have the test tray sitting on a plate (or something like it) to catch any of the purple cabbage extract.

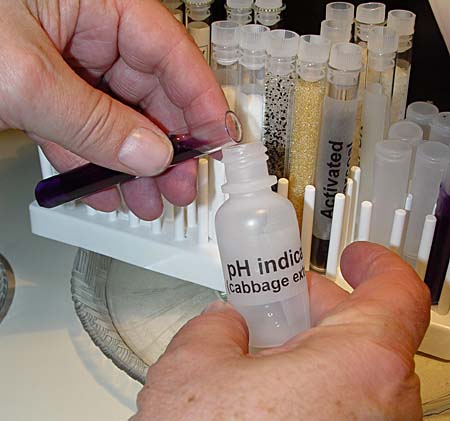

Just like the purified water bottle, the pH indicator (cabbage extract) bottle needs the nozzle replaced and the cap placed on it.
Take a picture of your pH indicator solution made of cabbage extract. Hold it so your face is visible too.
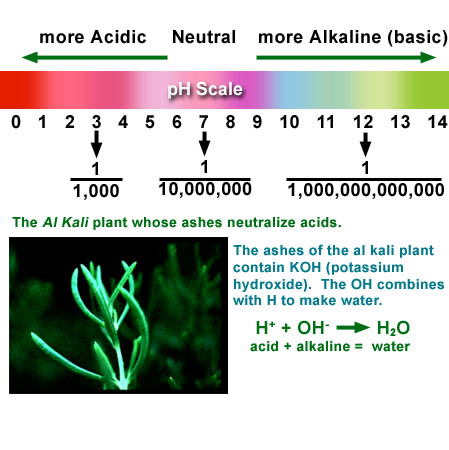
Red cabbage extract changes to many colors depending on
acidity. Acidity is measured on a pH scale. pH
stands for "potential for Hydrogen."
Acidity is caused by hydrogen atoms that have lost their electrons and
are roaming free in water [H+]. The scale goes from 0 to 14. The fractions
represent the concentration of these positively charged hydrogen atoms
[H+]. You can probably see the pattern. The numbers of the pH scale represent
the number of zeros behind the 1. In other words, they are exponents.
For example, 7 means ![]() or 10x10x10x10x10x10x10 or 10,000,000. Pure water has a pH of 7 because
it has an acid [H+] concentration of one over ten million. pH 3 has a
concentration of one thousandth. One thousandth is a larger number
than one ten millionth. Therefore the smaller the pH numbers the greater
the acid [H+] concentration.
or 10x10x10x10x10x10x10 or 10,000,000. Pure water has a pH of 7 because
it has an acid [H+] concentration of one over ten million. pH 3 has a
concentration of one thousandth. One thousandth is a larger number
than one ten millionth. Therefore the smaller the pH numbers the greater
the acid [H+] concentration.
The opposite of acidic is alkaline (or basic). Alkaline gets its name from the "al kali" plant whose ashes are capable of neutralizing acids. It does this because it contains KOH. The "OH" is called hydroxide and as the equation shows, it combines with the acid [H+] to form water.

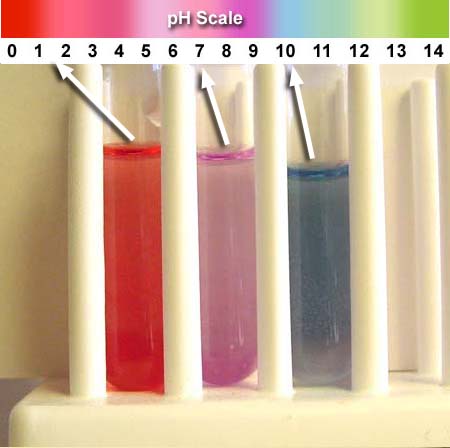
At the end of this experiment you will be comparing the colors of three test tubes. The left will have vinegar. The middle will have distilled water. The right will have baking soda in water. All three will have 3 drops of the red cabbage extract added to them. By looking at the color chart above, it shows the vinegar is in the 1 to 3 range. The distilled water is about 7 (as it should be) and the baking soda is around 10 meaning it is alkaline.
When solutions are alkaline, the [OH-] concentration is larger than the [H+] concentration. Water is neutral because the acid [H+] concentration is equal to the [OH-] concentration. Also, both of these are at very low concentrations (one ten millionth)





You can use white vinegar or lemon juice to check to see if your red cabbage extract really works.
Pour a little vinegar into the small 50mL beaker. 10 ml or less is plenty. You will only need about 3 ml to fill a test tube 1/4 of the way.
You pour into the beaker first because it has a small pouring spout, which makes it easier to pour into the test tube.

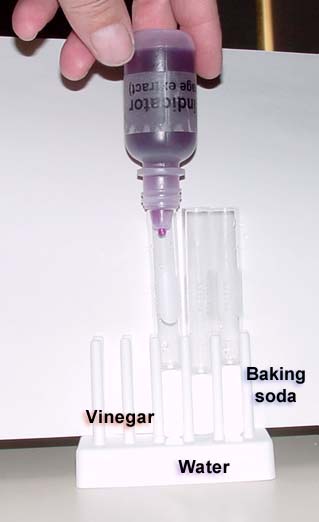
Place three drops of the red cabbage extract into each test tube. (if extra drop goes in, it's OK) The tubes with vinegar, water, and baking soda don't have to be in this order, but when they are, the acidic one is on the left, the neutral is in the middle and the basic (alkaline) one is on the right; which is the way the pH color scale is laid out.
After placing the drops in, you will need to pick up each test tube and swirl the liquid to get it to mix. Placing a sheet of white paper behind the test tubes will help the colors stand out.