Carbon Dioxide: Experiment #8: UV detection
and UV absorption
Using UV detection beads to test Sunglasses (and
auto glass)

Even though wearing sunglasses can make us look cool, the real reason to wear them is to protect the eyes. It's not the brightness that we need protection from, it's something we can't even see. The culprit is ultraviolet light, also called black light.



It is recommended that sunglasses block 95% to 99% of UV-A and UV-B light.
There's a wide array of sunglasses and nearly all of them say they block all or most of the UV light; however, how truthful are their claims?
This lab is to help you discover how good your sunglasses are at blocking UV light.

Your lab kit has a plastic packet that says "Ultraviolet Light Detection Cylinders". These white beads will turn color in the presence of UV light. The higher the intensity of UV light the brighter the color.


Your beads may vary from the ones shown. Some beads turn yellow, while others may turn orange, purple, red, turquoise, or some other color.

What you want to do is divide the beads into somewhat equal numbers and equal colors. In other words, try to have the same colors on both sides. Here I made sure that there were purple, turquoise, red, and orange on both sides.
We want the beads exposed directly to the sunshine to be about the same as the ones that will hide behind the sunglasses.


Here we see that the beads are starting to lose some of their color. This takes about one to two minutes.
I'm also using my hands to block any UV light that is coming from behind the sunglasses. Note that concrete and other structures can reflect the UV light. So I'm trying to only let light that comes through the sunglasses hit the test beads.
These are lightly tinted sunglasses, but that should not matter. UV absorption isn't coming from the dark tint. UV absorbers can be clear.

In about two to three minutes, reveal the the half that was covered by the sunglasses and compare the intensity of the colors. Do it quickly because the covered beads will quickly start to darken. Here I would estimate that the color intensity of the beads behind the sunglasses are bout 50% of that of the beads exposed to the sun. Unfortunately, this is far from 95% to 99% that is recommended.
I only buy glasses that says 100% UV blocking, so these aren't living up to that claim. On the other hand, I also buy cheap sunglasses, so that may be the reason.

Now I'm trying some darker pair of glasses. Again, the UV blocking doesn't need a dark tint.
You only need to test one pair of sunglasses, but feel free to test as many as you want.

Here again, it seems that I'm getting about a 50% blocking of the UV light. These were a cheap pair also, but it did say 100% UV blocking.
I've learned that some chemicals that block UV light will decompose. For example, sunscreen for your skin will start to breakdown and has to be replenished. It's possible that these glasses originally blocked 100% of UV light but as they were used the UV absorbers degraded.

Now I'm trying a pair of polarized sunglasses. Polarized light is light that is only vibrating in one direction (like up or down or side to side). When light reflects off of a horizontal surface like water or a windshield, the reflected light is mostly vibrating in one direction (probably up and down). Polarized sunglasses are made to allow light that is vibrating side to side to pass through blocking any light with up and down vibration. So it effectively blocks light that reflects off of horizontal surfaces. This can block UV light that is reflected but not UV light that isn't.

After waiting a couple of minutes, I flipped the glasses
over to see the beads that had the sunshine blocked by the polarized sunglasses.
Here again, it seems that the UV blocking is only around 50%. Polarized
glasses can do better if they have added UV absorbers. But blocking only
polarized light is only going to catch about 50%.

Here is another pair of sunglasses that is very lightly tinted. I like lightly tinted sunglasses because they let me see inside the car or items that are in shadows. I'm not bothered by bright light, I just want some UV protection. Let's see if these cheap sunglasses do that.
Here I just placed the beads behind the sunglasses.

Ouch. After a couple minutes to allow the beads to fade, these glasses show no UV protection even though when purchased, they were labeled as 100% UV blocking.
Again, they may have started with 100% UV blocking but quickly degraded to have no protection. Or they may have started with no protection and the manufacturer was simply telling a lie.


As I pulled the beads out from behind the RayBan glasses, the beads were nearly white. It showed that these glasses blocked basically 100% of the UV light. The instructor said they cost him a 100 dollars. I said it was worth it because you do a lot of outdoor activities and these glasses are protecting your eyes.
It seems that I may have to stop buying 5 to 10 dollar sunglasses, especially knowing they aren't protecting the eyes.

To show that the UV protection is possible with tinting, I've placed some beads behind the windshield of my truck. The bottom of the windshield is clear. It's only near the top of a windshield that is tinted.
Very quickly the beads started to fade meaning most of the UV light was blocked.

Here is a closeup of the beads behind the windshield. I'd say the beads show about a 75% blocking of UV light. I suspected the windshield is doing better than that but light is also coming in from the side windows bouncing off of the interior and hitting the beads.
The side and back windows are heavily tinted but let's see how well they block UV light.

Here is the inside of the passenger door with the beads on the inside.

After a few minutes the beads have not faded too much. I'd say the tinted windows are only blocking about 50% of the UV light.

Note that even in the shade, there is quite a bit of UV light bouncing around. So it's worth wearing sunglasses while even in the shade. It also appears that one could get a suntan while staying in the shade. The beads seem to indicated about 50% intensity of UV light even in the shade.

 As
with all labs, I need a picture of you doing the lab. Take a picture with
you holding or wearing the sunglasses that you were using. You can also
be holding the UV detection beads if you want.
As
with all labs, I need a picture of you doing the lab. Take a picture with
you holding or wearing the sunglasses that you were using. You can also
be holding the UV detection beads if you want.
Also, report your estimation of what percent of UV blocking you think your sunglasses was doing. If you know the brand and the cost, let me know. I'm in the market for some new sunglasses.
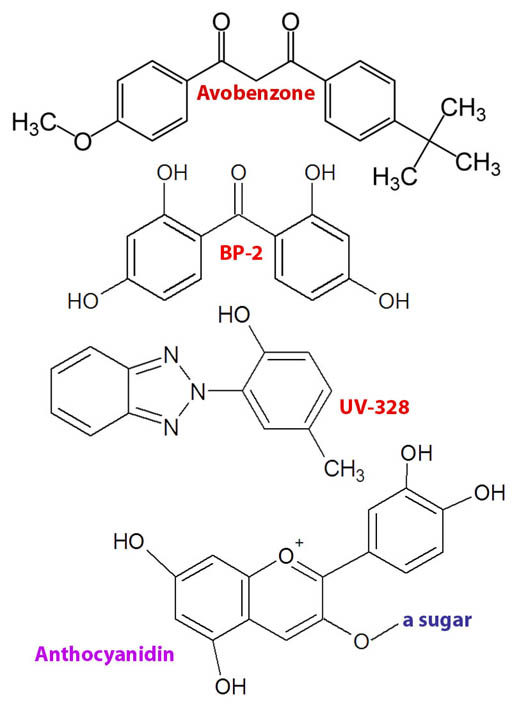
Here are some compounds that absorb UV light. The top one (Avobenzone) is a common ingredient in sunscreen. The next two (BP-2 & UV-238) are often placed in plastic, paint, or anything (like sunglasses) that needs some UV absorbing protection. The bottom one is a pigment in plants. If you've done the red cabbage lab quiz, you would have seen it. It is responsible for the color of some plants and is theorized to be a sunscreen for the plant.
Can you see a similarity with all of these light absorbers? They all have double bonds (double line connection) separated by a single bond (single line). Note: Double bonds are explained more in the Unhealthy Food tutorial.
This alternating arrangement of double and single bonds allows electrons to spread out over the whole length of these alternating bonds. What it boils down to is this allows the electrons to absorb lower frequencies of light, which includes visible and near UV light, the ones we are interested when discussing sunglasses.