Solubility is the ability of a gas, liquid, or solid to dissolve in water (or sometimes another liquid). A lava lamp, however, depends on the globs not being soluble. It also depends on the globs being about the same density as water. It took years to develop the right ingredients to make a lava lamp work.

The motto regarding solubility is "Like Dissolves Like." For example, to clean off grease, use something that is greasy like vegetable oil or butter. I had one student say he noticed potato chips cleaned his hands. It must have been the oil in the chips.

This child has a clean face now, but after eating the cotton candy, it won't be. To clean off cotton candy we know that water would good for that, not oil. Remembering "Like Dissolves Like" we guess that water and sugar must be alike in some way.
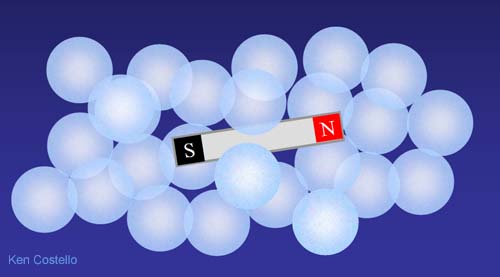
To understand how things dissolve, let's first look at something you are familiar with--- magnets. If you were to drop a small bar magnet into a group of marbles, you might suspect it to simply be sitting amidst a group of marbles. That is true, but now...
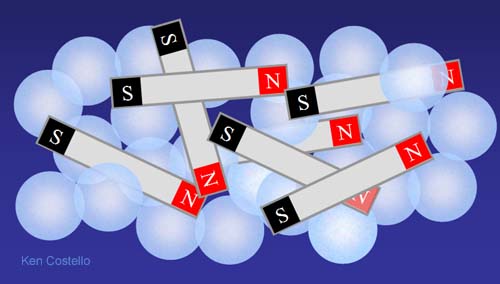
Let's throw in several of the small bar magnets. At first they will just be randomly mixed with the marbles. However, since magnets have north and south poles that attract each other, magnets will start to stick to other magnets. In a little while a different arrangement will occur.

The magnets pull toward each other and push the marbles out of the way as they come together. The glass marbles have no poles, so the magnets have no attraction to them. The magnets will find an arrangement that allows for opposite poles to get closer to each other and like poles to stay as far away from each other as possible. Also, since metal magnets are more dense than glass, gravity would pull them to the bottom.
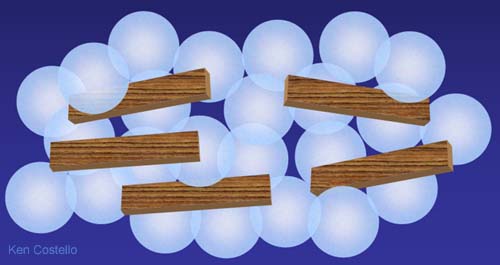
Instead of magnets, consider what happens when mixing in wood sticks. Wood has no magnetic poles so it doesn't attract other pieces of wood. The wood sticks would stay randomly mixed with the marbles.
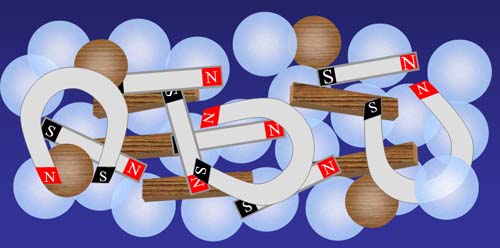
Now let's throw in a mixture of bar magnets, horseshoe magnets, wood sticks, and wood balls. As you might expect, only the items that have magnetic poles will try to reorganize.
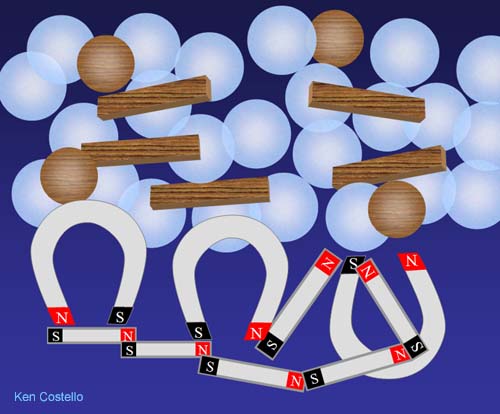
The items that have magnetic poles pull on each other and squeeze the items they don't attract out of the way. If the magnetic items are more dense than the non-magnetic items, they will end up below (like shown). If the magnets have the same density as the non-magnetic items, they end up as a ball in the middle. In every case, they separate themselves from the items that don't have any magnetic poles. It' not that the magnets repel the wood or glass, it's just they are very attracted to only things magnetic.
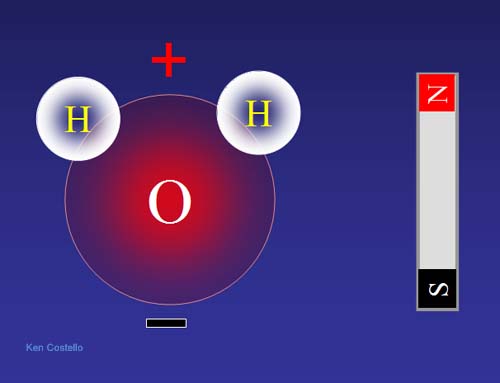
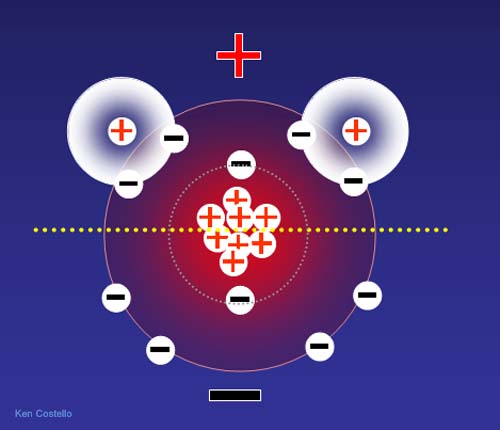
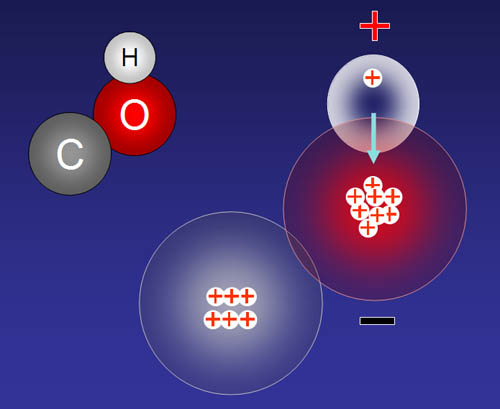
Why is water positive toward the hydrogen end? If we look at all of the protons (+) and electrons (-) of both hydrogen and oxygen, this is what we see. If you count the bottom half you get 4 protons and 5 electrons (a minus 1 net charge). If you count the top half, you get 6 protons and 5 electrons (a plus 1 net charge). Even though this is a simplification because electrons are in motion, we still get the idea why the top half (hydrogen half) is positive and the bottom half (oxygen only half) is negative.
Molecules like water that have plus and negative poles (like magnetic poles) are called POLAR. Molecular that do not have any negative or positive poles are called nonpolar.


Water is always trying to pull itself into a tight ball as long as there is nothing nearby that has a charge on it. Therefore, this surface is not repelling water; it’s simply not attracting it and keeping water from doing what it does naturally.
Again, wax on a car's paint doesn't repel water, it simply
isn't attracting it. Water pulls itself into beads on
its own.

Since water pulls itself together strongly to form drops, raindrops are possible. I don't know of any other liquid that could fall from the sky and keep itself in drops. Most liquids would just break up into tiny drops or fog.
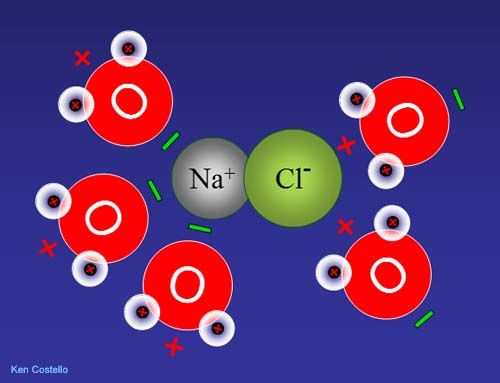
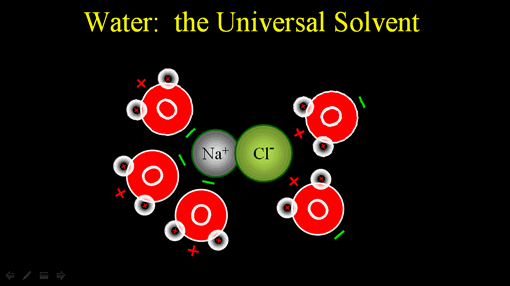
Let's return to water's ability to dissolve many substances. The substances that water is good dissolving are those that have a charge. Here is sodium chloride (table salt). Sodium has a net plus charge because chlorine has taken one of sodium's electrons. This makes chlorine negative because it has an extra electron.
The negative end of water pulls on the sodium atom and the positive end of other water molecules pull on the negatively charged chlorine.
Since unlike charges attract, the negative end of water will be attracted to the positive sodium ion. The positive end of water will be attracted to the negative chloride ion.
Since water is always in motion, it will pull on the ionic compound and move the ions away from each other. This dissolves the ionic compound. (Roll cursor over image to see animation).

Salts, like NaCl (table salt) are basically a metal combined with a non-metal. See the below Periodic Table of the Elements which is color-coded for metals versus non-metals.
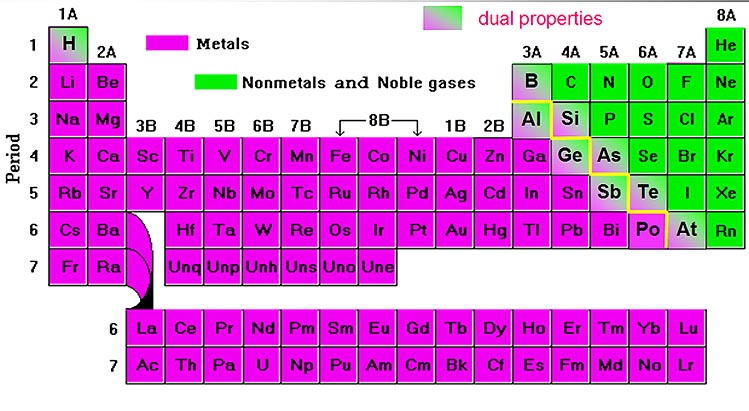
The elements colored with green are non-metals and the ones in purple are metals. The ones that blend from green to purple have qualities of both a metal and non-metal. In the upper right you will see "7A." Below that are the elements called "halogens." Halo means salt, and gen means generate. So halogen means salt generator. The main elements in this column are fluorine, chlorine, bromine, iodine. When these come in contact with a metal, they quickly turn the metal into a powdery salt. The metal will have a positive charge because the halogen pulls an electron off. The halogen then becomes negatively charged. As you know water is attracted to these charges and will try to pull it apart. A lot of metals are toxic, so if they are in salt form, they may dissolve (pulled apart by water) and contaminate drinking water.
Another class of compounds that are soluble in water are nitrates. The sources of nitrates are shown below - barnyard and human waste plus synthesized fertilizers.


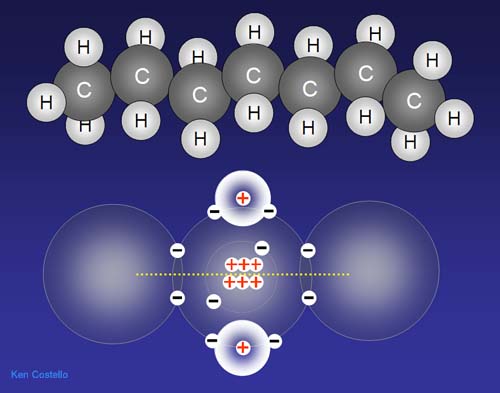


A compound added to gasoline to help it burn more cleanly
is Methyl Tertiary Butyl Ether, abbreviated as MTBE. Notice the formula
shows a carbon attached to an oxygen attached to another carbon. Like
the above diagram, the oxygen will pull electrons closer to it. This makes
the oxygen partially negatively charged. But it's enough charge for water
to pull on it and make it dissolve in water. Unfortunately, many gasoline
storage tanks leak and the MTBE is easily dissolved by rain water, which
can carry the MTBE into the drinking water supplies. Over 20,000 tanks
are estimated as leaking in the state of Virginia alone.
MTBE is a known to cause cancer in animals and possibly humans. But even extremely small amounts that would not be dangerous can add a turpentine taste to water and make the water undrinkable just because of bad taste. It's ironic that the oxygen atom that provides oxygen for cleaner combustion is also the reason that MTBE is water soluble and a threat to drinking water.